c) Compound A below undergoes Dieckmann cyclization to give compound B in the presence of a base. Dieckmann EtO OEt cyclisation OEt Base CH3 A i) Give the appropriate base for the above reaction and draw the resonance structures of the enolate ions derived from compound A. ii) If compound B above is reacted with NaBH4, draw the structure of the reduced product. Explain a reason for your choice of the product.
c) Compound A below undergoes Dieckmann cyclization to give compound B in the presence of a base. Dieckmann EtO OEt cyclisation OEt Base CH3 A i) Give the appropriate base for the above reaction and draw the resonance structures of the enolate ions derived from compound A. ii) If compound B above is reacted with NaBH4, draw the structure of the reduced product. Explain a reason for your choice of the product.
Chapter14: Conjugated Compounds And Ultraviolet Spectroscopy
Section14.SE: Something Extra
Problem 50AP: -Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the...
Related questions
Question
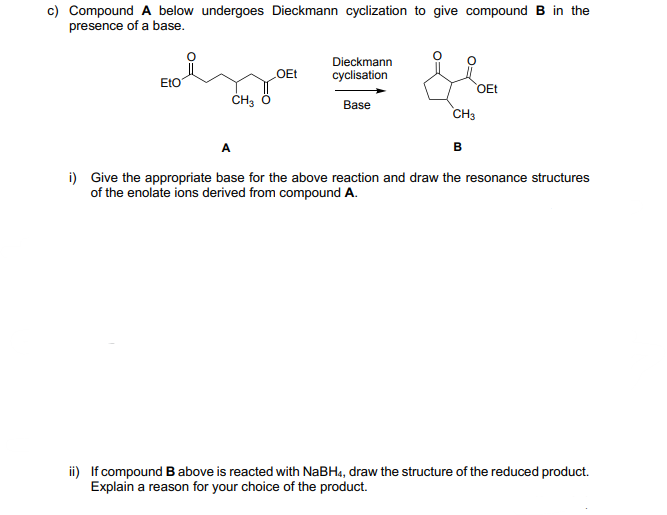
Transcribed Image Text:c) Compound A below undergoes Dieckmann cyclization to give compound B in the
presence of a base.
Dieckmann
EtO
OEt
cyclisation
OEt
Base
CH3
A
i) Give the appropriate base for the above reaction and draw the resonance structures
of the enolate ions derived from compound A.
ii) If compound B above is reacted with NaBH4, draw the structure of the reduced product.
Explain a reason for your choice of the product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning