c) How many grams of the excess reactant remain after the limiting reactant is completely consumed? d) If 0.723 g of sodium citrate was obtained in this reaction, then what is the percentage yield?
c) How many grams of the excess reactant remain after the limiting reactant is completely consumed? d) If 0.723 g of sodium citrate was obtained in this reaction, then what is the percentage yield?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 113AE: Gold is produced electrochemically from an aqueous solution of Au(CN)2 containing an excess of CN....
Related questions
Question
can you help me please?
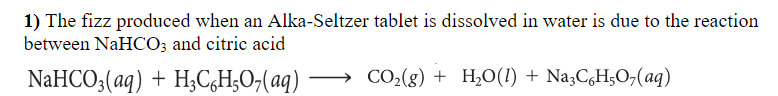
Transcribed Image Text:1) The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction
between NaHCO; and citric acid
NaHCO;(aq) + H,C,H;O,(aq)
→ CO2(g) + H¿O(!) + Na3CgH;O,(aq)

Transcribed Image Text:c) How many grams of the excess reactant remain after the limiting reactant is
completely consumed?
d) If 0.723 g of sodium citrate was obtained in this reaction, then what is the percentage
yield?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning