(c) (i) Identify the product that would be obtained by the reaction of benzaldehyde (PHCHO) with isopropylamine (Me2CHNH2) under acidic conditions. Draw a full reaction mechanism for its formation. (ii) Explain why piperidine (F) will not react with benzaldehyde in the same way as isopropylamine.
(c) (i) Identify the product that would be obtained by the reaction of benzaldehyde (PHCHO) with isopropylamine (Me2CHNH2) under acidic conditions. Draw a full reaction mechanism for its formation. (ii) Explain why piperidine (F) will not react with benzaldehyde in the same way as isopropylamine.
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 35MP: One step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to...
Related questions
Question
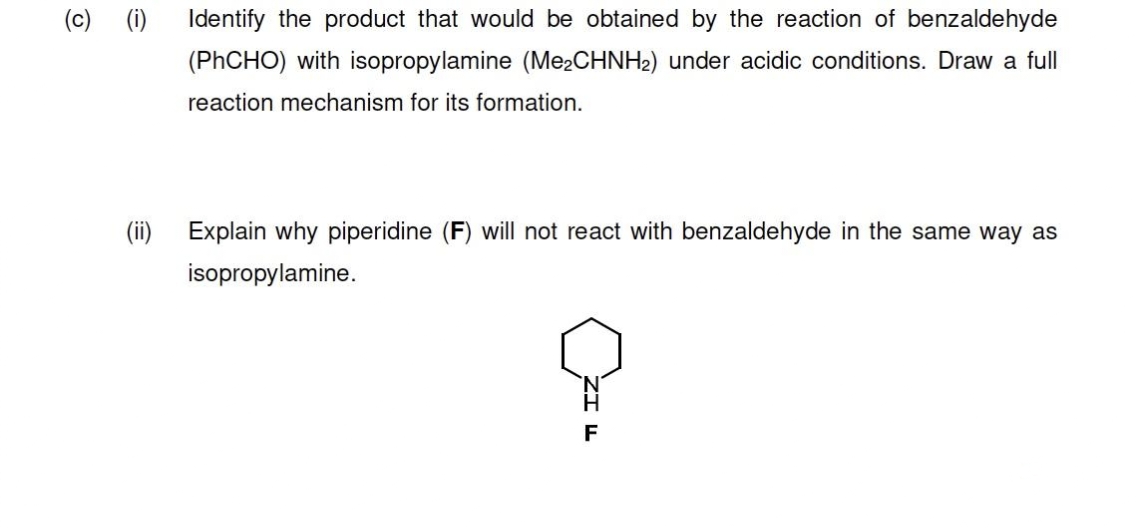
Transcribed Image Text:(c) (i)
Identify the product that would be obtained by the reaction of benzaldehyde
(PHCHO) with isopropylamine (Me¿CHNH2) under acidic conditions. Draw a full
reaction mechanism for its formation.
(ii) Explain why piperidine (F) will not react with benzaldehyde in the same way as
isopropylamine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning