(c) On the attached reference sheet, locate the value of Ksp for cobalt (II) arsenate at 25°C. Determine the equilibrium concentrations, in mol L-', of each ion in the solution at 25°C. Be sure to show your work. (d) Calculate the mass, in mg, of cobalt (II) arsenate that is present in 50.0 mL of a saturated solution at 25°C. (e) Which salt, cobalt (II) arsenate or zinc arsenate has the greater value of [AsO4* ] at equilibrium at 25°C? Justify your answer. *Note – you do not need to use a calculation. (The value of Kp for zinc arsenate at this temperature is 2.8 × 10 28.)
(c) On the attached reference sheet, locate the value of Ksp for cobalt (II) arsenate at 25°C. Determine the equilibrium concentrations, in mol L-', of each ion in the solution at 25°C. Be sure to show your work. (d) Calculate the mass, in mg, of cobalt (II) arsenate that is present in 50.0 mL of a saturated solution at 25°C. (e) Which salt, cobalt (II) arsenate or zinc arsenate has the greater value of [AsO4* ] at equilibrium at 25°C? Justify your answer. *Note – you do not need to use a calculation. (The value of Kp for zinc arsenate at this temperature is 2.8 × 10 28.)
Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.8QAP
Related questions
Question
The solubility product constants for Cobalt (II) Arsenate and Zinc Arsenate for the mentioned reference sheet for part {c} are attached below. Also, please solve from parts c) to e) as parts a) and b) have already been solved. Thank you!
![Question 1
Consider the sparingly soluble salt cobalt (II) arsenate.
(a) Write a balanced chemical equation for the dissolution of cobalt (II) arsenate in distilled water.
(b) Write the expression for the solubility-product constant, Ksp, for cobalt (II) arsenate.
(c) On the attached reference sheet, locate the value of Ksp for cobalt (II) arsenate at 25°C.
Determine the equilibrium concentrations, in mol L-1, of each ion in the solution at 25°C. Be
sure to show your work.
(d) Calculate the mass, in mg, of cobalt (II) arsenate that is present in 50.0 mL of a saturated
solution at 25°C.
(e) Which salt, cobalt (II) arsenate or zinc arsenate has the greater value of [AsO4³] at equilibrium
at 25°C? Justify your answer. *Note – you do not need to use a calculation. (The value of Ksp
for zinc arsenate at this temperature is 2.8 × 10-28.)
-](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ffbbfdd36-764e-4885-9d97-965ca07081b8%2Facf3a54e-1f57-4b99-99c7-3341bad514f9%2Fk70qgt_processed.png&w=3840&q=75)
Transcribed Image Text:Question 1
Consider the sparingly soluble salt cobalt (II) arsenate.
(a) Write a balanced chemical equation for the dissolution of cobalt (II) arsenate in distilled water.
(b) Write the expression for the solubility-product constant, Ksp, for cobalt (II) arsenate.
(c) On the attached reference sheet, locate the value of Ksp for cobalt (II) arsenate at 25°C.
Determine the equilibrium concentrations, in mol L-1, of each ion in the solution at 25°C. Be
sure to show your work.
(d) Calculate the mass, in mg, of cobalt (II) arsenate that is present in 50.0 mL of a saturated
solution at 25°C.
(e) Which salt, cobalt (II) arsenate or zinc arsenate has the greater value of [AsO4³] at equilibrium
at 25°C? Justify your answer. *Note – you do not need to use a calculation. (The value of Ksp
for zinc arsenate at this temperature is 2.8 × 10-28.)
-
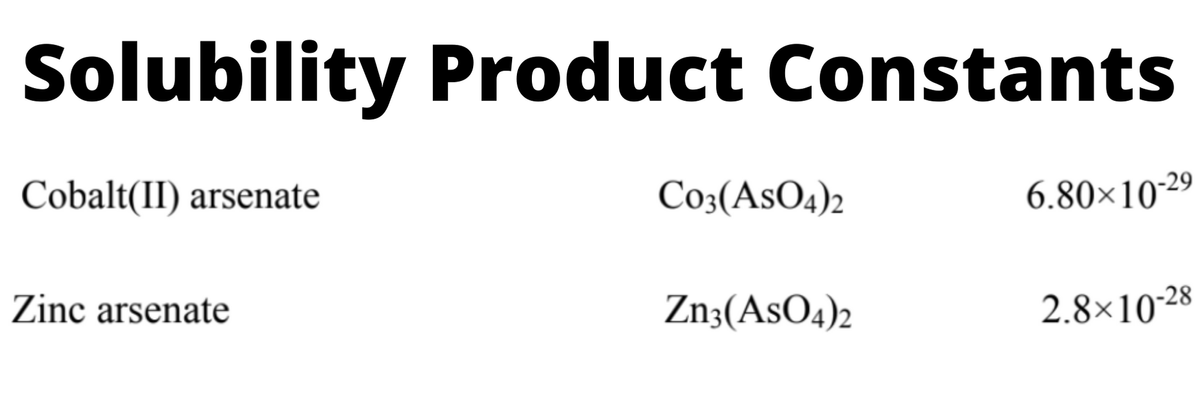
Transcribed Image Text:Solubility Product Constants
Cobalt(II) arsenate
Co3(AsO4)2
6.80×10-29
Zinc arsenate
Zn3(AsO4)2
2.8×10-28
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning