Determine the hybridization at each of the 3 labeled atoms.
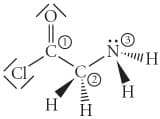
atom labelled 1.
Since here C is the centre atom and it has 4 valence electron
since 3 of the valence electrons are used in making 3 sigma bonds and 1 is used in making pi bond
Hence bonding electron pair = 3
and since no electron is remaining in C => non bonding electron pair = 0
Hence total number of hybridised orbitals = 3+0 = 3
hence hybridisation = sp2
atom labelled 2.
Since here C is the centre atom and it has 4 valence electron
since all 4 of the valence electrons are used in making 4 sigma bonds
Hence bonding electron pair = 4
and since no electron is remaining in C => non bonding electron pair = 0
Hence total number of hybridised orbitals = 4+0 = 4
hence hybridisation = sp3
Step by step
Solved in 3 steps




