(c). In the laboratory, you wish to determine the reaction order and rate constant for the following thermal decomposition reaction: 2 AB2 - A2 + 2 B2 (i). Identify the data you will collect. (ii). Explain how you would use these data to determine whether the reaction is zero order, first order or second order and the value of the rate constant.
(c). In the laboratory, you wish to determine the reaction order and rate constant for the following thermal decomposition reaction: 2 AB2 - A2 + 2 B2 (i). Identify the data you will collect. (ii). Explain how you would use these data to determine whether the reaction is zero order, first order or second order and the value of the rate constant.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter14: Chemical Equilibirum
Section: Chapter Questions
Problem 14.27QP
Related questions
Question
Kindly answer question c
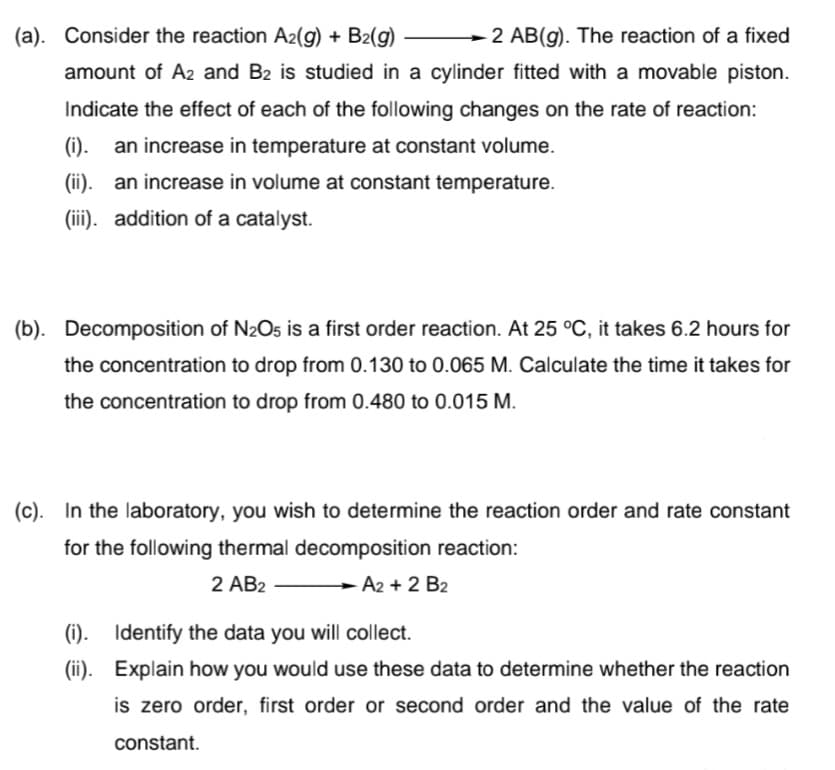
Transcribed Image Text:(a). Consider the reaction A2(g) + B2(g)
2 AB(g). The reaction of a fixed
amount of A2 and B2 is studied in a cylinder fitted with a movable piston.
Indicate the effect of each of the following changes on the rate of reaction:
(i). an increase in temperature at constant volume.
(ii). an increase in volume at constant temperature.
(iii). addition of a catalyst.
(b). Decomposition of N2O5 is a first order reaction. At 25 °C, it takes 6.2 hours for
the concentration to drop from 0.130 to 0.065 M. Calculate the time it takes for
the concentration to drop from 0.480 to 0.015 M.
(c). In the laboratory, you wish to determine the reaction order and rate constant
for the following thermal decomposition reaction:
2 AB2
A2 + 2 B2
(i).
Identify the data you will collect.
(ii). Explain how you would use these data to determine whether the reaction
is zero order, first order or second order and the value of the rate
constant.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning