Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
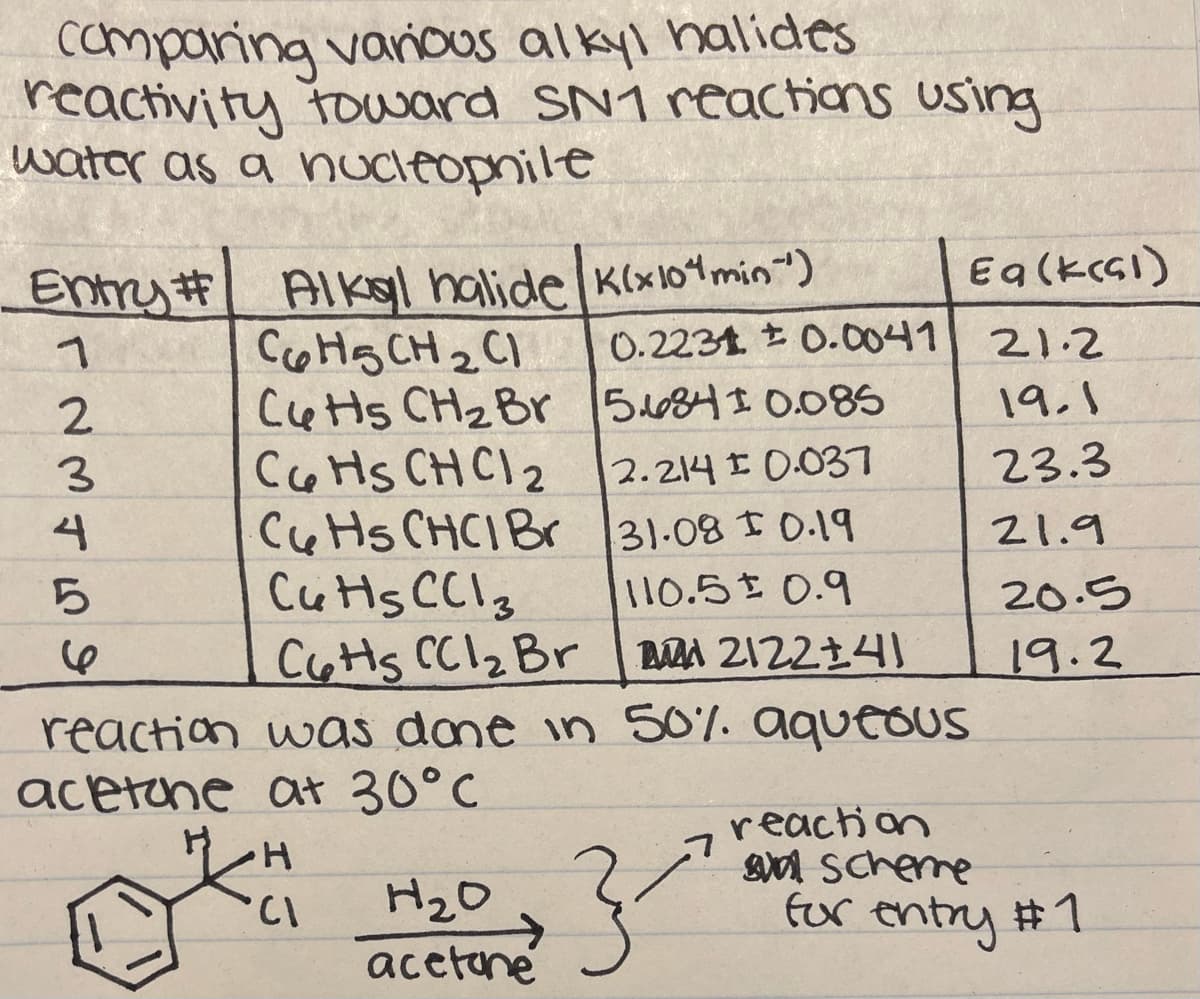
Transcribed Image Text:camparing vanious alkyl halides
reactivity toward SN1 reactions using
water as a nucleopnile
Entry # Alko| halide K(x101min")
0.2231 t 0.0041
Eq(kcsI)
COHS CH 2 C1
Cetts CH2Br
coHs CHCI 2
Ce Hs CHCIBr
Cuts CCI3
CoHs CC12 Br A 212214)
21.2
2.
5684I 0.085
19.1
2.214 E 0.037
23.3
31.08 1 0.19
110.5 0.9
4
21.9
20.5
19.2
reaction was dame in 50%. aqueOUS
acetune at 30°C
reaction
n scherre
fur entry #1
H2O
->
acetane
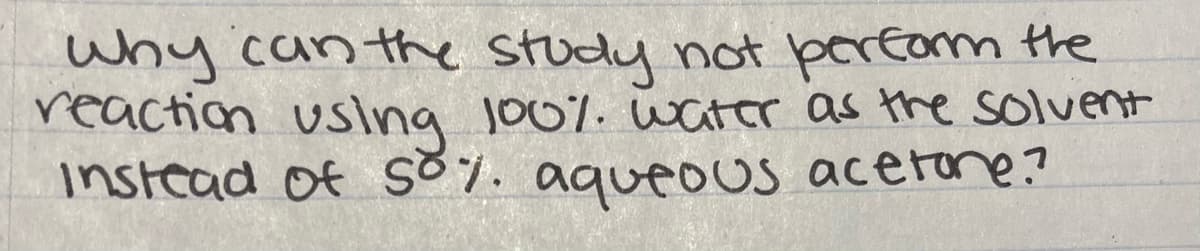
Transcribed Image Text:why can the study not perfom the
reaction
using 100%. water as the solvent
instead of S7. aqueous acerone?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

