Calcium carbonate decomposes to lime according to the following reaction: CaCO3(s) = CaO(s) + CO2(g) %3D What is the entropy change of the reaction at standard conditions? Substance AH° (kJ/mol) S° U/mol.K) Cp U/mol.K) AHm U/mol) Cu (s) 33.4 22.65+0.00628T 13000 @ 1356 K Cu (1) 41.6 31.40 CaCO, (s) -1207 88.7 104.57+0.02193T-2595000/T CaO(s) -635.5 38.2 49.95+0.00489T-352000/T Co2(g) O2(g) -393.5 213.6 22.24+0.0598T-349900/T 205.1 33.44 -167.4 93.1 83.6
Calcium carbonate decomposes to lime according to the following reaction: CaCO3(s) = CaO(s) + CO2(g) %3D What is the entropy change of the reaction at standard conditions? Substance AH° (kJ/mol) S° U/mol.K) Cp U/mol.K) AHm U/mol) Cu (s) 33.4 22.65+0.00628T 13000 @ 1356 K Cu (1) 41.6 31.40 CaCO, (s) -1207 88.7 104.57+0.02193T-2595000/T CaO(s) -635.5 38.2 49.95+0.00489T-352000/T Co2(g) O2(g) -393.5 213.6 22.24+0.0598T-349900/T 205.1 33.44 -167.4 93.1 83.6
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.33PAE: Limestone is predominantly CaCO3, which can undergo the reaction CaCO3(s)CaO(s)+CO2(g). We know from...
Related questions
Question
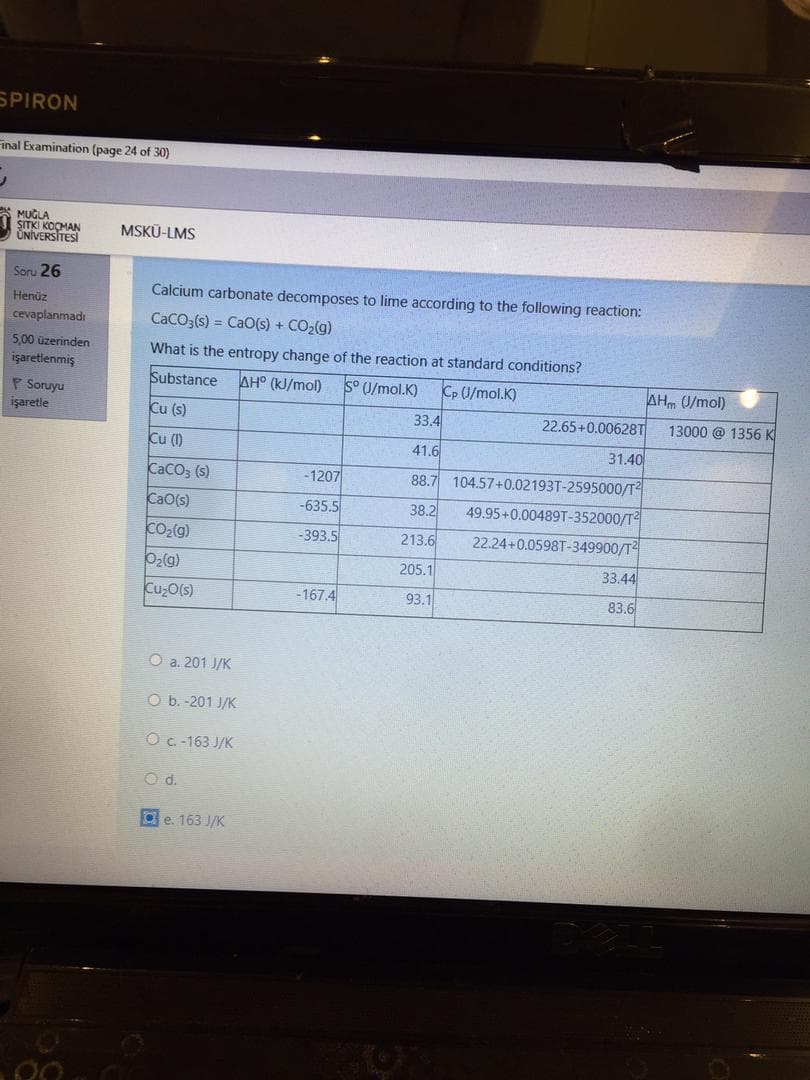
Transcribed Image Text:SPIRON
Final Examination (page 24 of 30)
尝MUCLA
SITKI KOCMAN
UNİVERSİTESİ
MSKÜ-LMS
Soru 26
Calcium carbonate decomposes to lime according to the following reaction:
Henüz
cevaplanmadı
CaCO3(s) = CaO(s) + CO2(g)
5,00 üzerinden
işaretlenmiş
What is the entropy change of the reaction at standard conditions?
Substance
AH° (kJ/mol)
S° U/mol.K)
Cp U/mol.K)
AHm U/mol)
P Soruyu
işaretle
Cu (s)
33.4
22.65+0.00628T
13000 @ 1356 K
Cu (1I)
41.6
31.40
CaCO, (s)
-1207
88.7
104.57+0.02193T-2595000/T
CaO(s)
-635.5
49.95+0.00489T-352000/T
38.2
CO2(g)
-393.5
213.6
22.24+0.0598T-349900/T
02(g)
205.1
33.44
Cu,O(s)
-167.4
93.1
83.6
O a. 201 J/K
O b. -201 J/K
O C.-163 J/K
O d.
e. 163 J/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning