Calculate AG° for the following reaction, using the provided information. 2 CoН6) + 15 02(9) — 12 СО2(9) + 6 H20(9) kJ AG°;(CO2(9) = -394.6 "/mol kJ AG°,(H20(9) = -228.4 mol kJ AG°;(H201) = -237.2 "/mol kJ mol AG°;(C6H6) = = -121.7
Calculate AG° for the following reaction, using the provided information. 2 CoН6) + 15 02(9) — 12 СО2(9) + 6 H20(9) kJ AG°;(CO2(9) = -394.6 "/mol kJ AG°,(H20(9) = -228.4 mol kJ AG°;(H201) = -237.2 "/mol kJ mol AG°;(C6H6) = = -121.7
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 47QAP: Plants synthesize carbohydrates from CO2 and H2O by the process of photosynthesis. For example,...
Related questions
Question
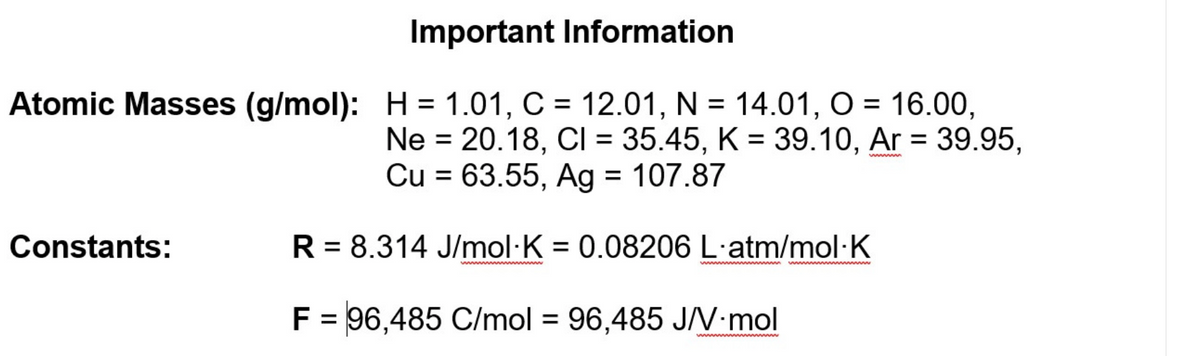
Transcribed Image Text:Important Information
Atomic Masses (g/mol): H = 1.01, C = 12.01, N = 14.01, O = 16.00,
%D
Ne = 20.18, CI = 35.45, K = 39.10, Ar = 39.95,
Cu = 63.55, Ag = 107.87
%3D
%3D
wwm
Constants:
R = 8.314 J/mol·K = 0.08206 L·atm/mol·K
%3D
ww m
www m
F = 96,485 C/mol = 96,485 J/V•mol
%3D
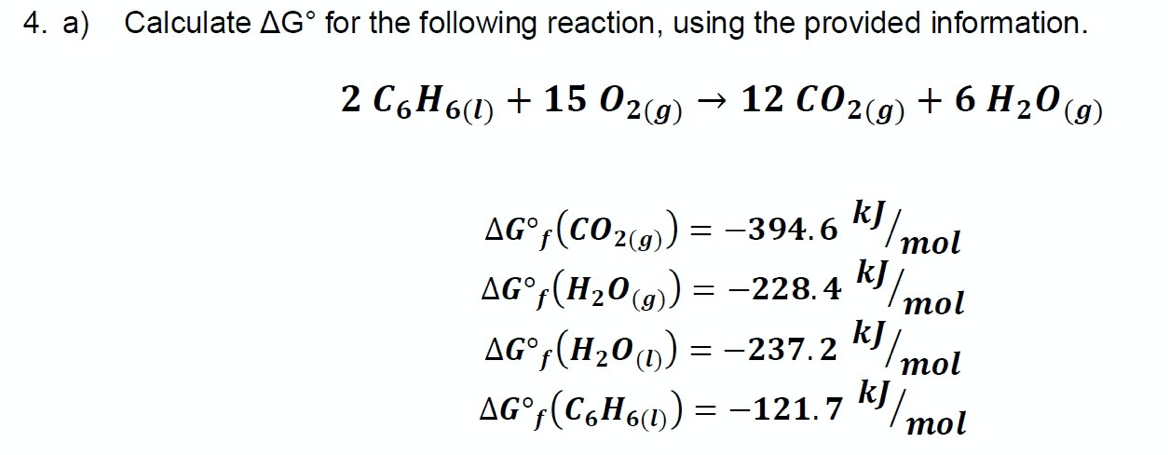
Transcribed Image Text:4. a) Calculate AG° for the following reaction, using the provided information.
2 C6H6(1) + 15 02(9)
→ 12 CO2(9) + 6 H20 (g)
AG°;(CO2(9) = -394. 6
"/mol
AG°(H20(9) = -228.4
(g)
mol
AG°,(H20u) = -237.2 k
mol
kJ
AG°,(C,H6») = -121.7 K/
тol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning