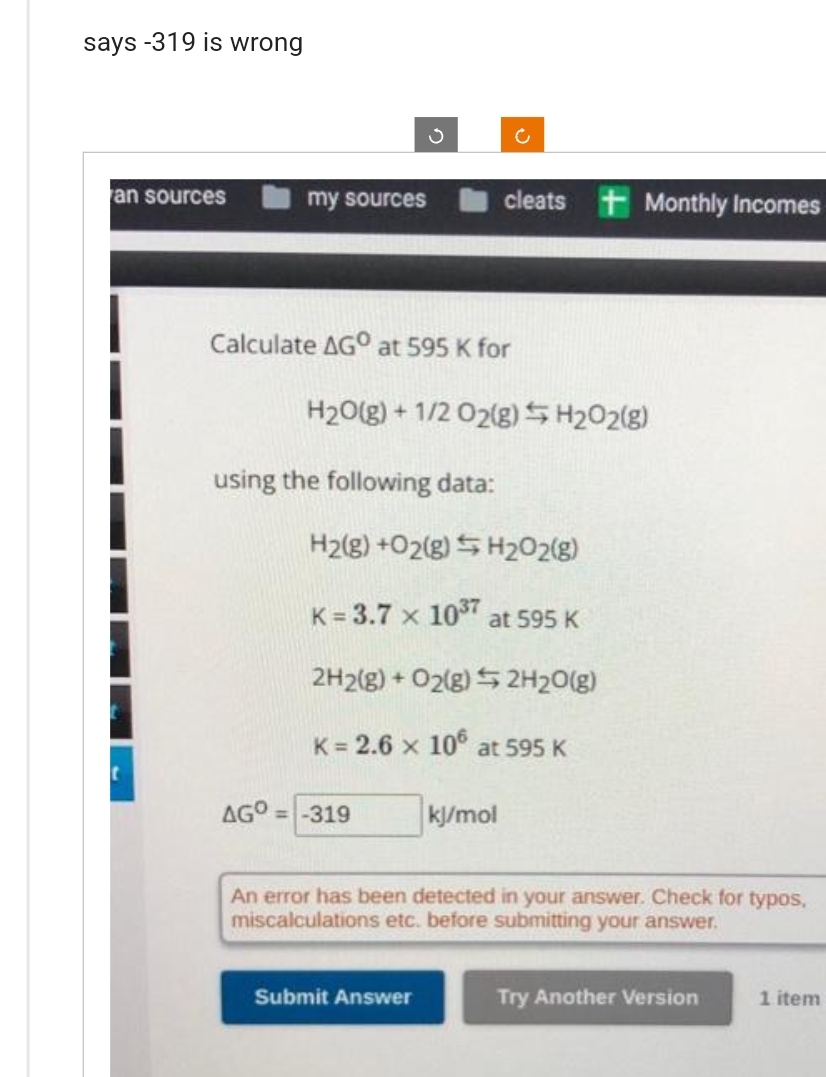
Calculate AGO at 595 K for H₂O(g) + 1/2 O2(g) H₂O2(g) using the following data: H2(g) +O2(g) H₂O2(g) K= 3.7 x 10³7 at 595 K 2H2(g) + O2(g) 2H₂O(g) K= 2.6 x 106 at 595 K AGO = -319 kj/mol
Calculate AGO at 595 K for H₂O(g) + 1/2 O2(g) H₂O2(g) using the following data: H2(g) +O2(g) H₂O2(g) K= 3.7 x 10³7 at 595 K 2H2(g) + O2(g) 2H₂O(g) K= 2.6 x 106 at 595 K AGO = -319 kj/mol
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 137MP: A gaseous hydrocarbon reacts completely with oxygen gas to form carbon dioxide and water vapour....
Related questions
Question
Mm.44.
Subject:- Chemistry
Transcribed Image Text:says -319 is wrong
an sources
my sources
S
Calculate AGO at 595 K for
using the following data:
H₂O(g) + 1/2O2(g) H₂O2(g)
AGO=-319
c
cleats Monthly Incomes
H2(g) +O2(g) H₂O2(g)
K = 3.7 x 10³7
Submit Answer
2H2(g) + O2(g) 2H₂O(g)
K = 2.6 x 106 at 595 K
at 595 K
kj/mol
An error has been detected in your answer. Check for typos,
miscalculations etc. before submitting your answer.
Try Another Version
1 item
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning