Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 93AP
Related questions
Question
100%
Calculate for the theoretical and percent yield. Please show detailed calculations.
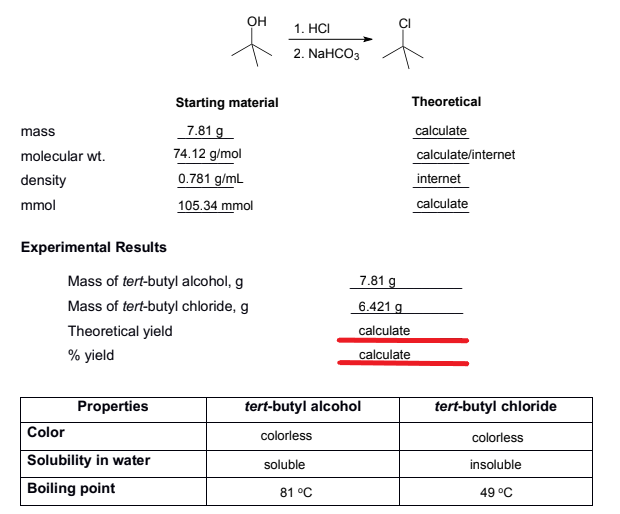
Transcribed Image Text:ОН
1. HCI
2. NaHCO3
Starting material
Theoretical
7.81 g
calculate
mass
74.12 g/mol
0.781 g/mL
105.34 mmol
molecular wt.
calculate/internet
density
internet
mmol
calculate
Experimental Results
Mass of tert-butyl alcohol, g
7.81 g
Mass of tert-butyl chloride, g
6.421 g
Theoretical yield
calculate
% yield
calculate
Properties
tert-butyl alcohol
tert-butyl chloride
Color
colorless
colorles
Solubility in water
soluble
insoluble
Boiling point
81 °C
49 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax