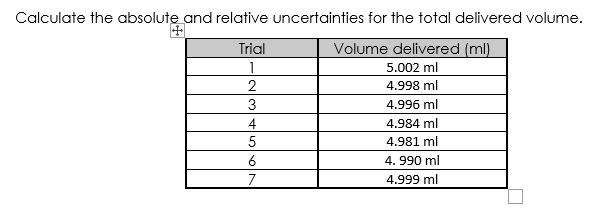
Calculate the absolute and relative uncertainties for the total delivered volume. Trial Volume delivered (ml) 1 5.002 ml 2 4.998 ml 4.996 ml 3 4 5 6 7 4.984 ml 4.981 ml 4.990 ml 4.999 ml
Q: 16.30 Draw structural formulas for the hemiacetal and then the acetal formed from each pair of…
A: Here we are required to hemiacetal and acetal formed during the reaction.
Q: Which one of the following statements best describes resonance? The actual substance interconverts…
A:
Q: 4. The following molecule was subjected to two different conditions. Provide the product in each…
A: Given reactants are amines . Both reactions are amine elimination reactions. Amine on…
Q: The proton NMR for the following compound was run. Assign peaks and integration for each proton.…
A: NMR spectroscopy of organic compounds.
Q: Sketch a graph for the solubility of potassium nitrate and cerium sulfate in water as a function of…
A: We need to construct a graph for the solubility of (a) potassium nitrate and (b) cerium sulfate in…
Q: A chromatogram gives an ideal Gaussian peak with tp = 8.30 min and w1/2 = 0.18 min. How many…
A: It is given that a chromatogram gives a Gaussian peak with tR = 8.20 min and w1/2 = 0.18 min. We…
Q: carbon monoxide(g) + oxygen(g) → carbon dioxide (g) at is the maximum mass of carbon dioxide that…
A:
Q: Why is the 4-methoxyphenyl acetate organic layer subjected to washes with saturated sodium…
A: Separatory funnel extraction is used to separate two immiscible liquids from the mixture. Upper and…
Q: chemical reaction takes place inside a flask submerged in a water bath. The water bath contains…
A:
Q: Which of the following metals would be expected to have the smallest atomic radius? A) Cesium (Cs)…
A: An atomic radius is defined as the half of the distance between the adjacent atoms of the same…
Q: Classify the solution that results, if you mix 50.0 mL of 0.20 M KOH(aq) and 50.0 mL of 0.20 M…
A:
Q: 9. Provide a synthetic route for each transformation below. مير 'OH ΕΙΟ H₂CO OH . NH2
A: These compounds can be synthesized by ozonalysis, wittig reaction, Baylis-hillman reaction,…
Q: O E What type of lipid is produced by the reaction shown here? (CH₂)14CH3 -(CH₂)14CH3 + excess NaOH…
A: The above reaction will form soap as a product. As fats with sodium hydroxide react to give soaps as…
Q: 0 Question 12 You combine 100. mL of 0.100 M Fe(NO3)2 and 100. mL of 0.125 M Na₂CO3. Does a…
A: Formation of Precipitate using the solubility product and reaction quotient.
Q: (I pn Ph hv сия, вст
A: when light falls photochemical reaction take place
Q: (P) (ә) HO ОН ОН Cl
A: (d) The synthesis of (2R,3S)-heptane-1,2-diol from propane can be carried out by using the following…
Q: Use tabulated heats of formation to determine the standard heats of the following reactions in kJ,…
A:
Q: The rate constant k for a certain reaction is measured at two different temperatures: temperature…
A:
Q: All normal matter that we know, is made of elements. 90 occur naturally and another 26 are man-made…
A: Big Bang theory is rooted to the origin and the evolution of the universe. It provides important…
Q: Draw the product of the Negishi coupling reaction that is expected when the following organic…
A: Given reaction is Negishi coupling reaction. Here aryl halide react with organozinc compound to…
Q: When 4.142 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 12.12 grams…
A:
Q: 1- what if B = benzene, A = methanol, what are the dominant interactions between A and B, B and B…
A: (1) To identify the dominant interaction between A and A, B and B and A and B and their dependence…
Q: What is the equilibrium vapor pressure of mercury at 100 °C according to the equation Hg(1) Hg(g) if…
A:
Q: How many moles of arsenic are there in 6.50 g of arsenic?
A:
Q: Draw three different resonance structures of chlorate, CIO,? Explain which one would be the most…
A: Chlorate ion , ClO3- has eight different Lewis structures . Out of these eight only three structures…
Q: A copper atom has a mass of 1.06 × 10 22 g and a penny has a mass of 2.5 g. Use this information to…
A:
Q: Draw the structure for chloric acid, HClO3. Optimize formal charges.
A: Name of compound - Chloric acid Molecular formula - HClO3 Given compound is neutral compound with…
Q: Although water is the most common hydrogen-oxygen compound, hydrogen and oxygen form another…
A: In earlier days, hydrogen peroxide(H2O2) was used as an antiseptic which can kill the germs by…
Q: Write the electron configuration using the half shell method and electron dot formula of…
A:
Q: Enter your answer in the provided box. A rock sample originally contains 4.79 mg of 238U. How much…
A: The question belongs to Nuclear Chemistry. We need to find how much lead will be in the rock sample…
Q: Figure A and Figure B represent examples of different types of chemical bonding. Identify the…
A: Ionic bond: These bonds are formed by the transfer of electrons from one atom to another. Covalent…
Q: Write the balanced nuclear equations for the following reactions and identify X
A: The general representation of an element is - XZAWhere,…
Q: A 2.20 L container at 53.6 °C contains 6.21g N2O3(g). The N2O3 gas decomposes completely,…
A: Given : Volume of vessel = 2.20 L Temperature = 23.6 °C = 296.6 K Mass of N2O3 = 6.21 g
Q: List the steps of the copper cycle. Which reactions are acid-base, and which are precipitation?
A: In the copper cycle there are following 6 steps
Q: What are the two main disadvantages of soap versus detergents? Illustrate your answer with chemical…
A: •SOAP :- Soap is long chain fatty acids salt of potassium or sodium. •DETERGENT:- Detergent is long…
Q: Consider the following data on some weak acids and weak bases: name acid nitrous acid hydrocyanic…
A:
Q: The three-dimensional structure of a generic molecule is given. Identify the axial and equatorial…
A:
Q: A brick has a mass of 4.0 kg and the Earth has a mass of 6.0 × 10²7 g. Use this information to…
A: 1mole brick = 6.022x1023 bricks so to find the mass of 1mole brick, we just need to calculate the…
Q: You would like to produce a gold-plated coin by plating gold onto a penny 1.90 cm in diameter. How…
A: First we need to calculate the total volume of gold deposited on coin . Then using density we would…
Q: Green plants use light from the Sun to drive photosynthesis, a chemical reaction in which liquid…
A: The unbalanced chemical reaction of photosynthesis is: CO2g+H2Og→C6H12O6aq+O2
Q: Question 1 Based on the solubility rules, which compounds do you expect to be highly soluble in…
A:
Q: ¹H NMR (CDC13): 8 7.1 (m, 5H), 2.5 (m, 1H), 1.6 (m, 2H), 1.22 (d, 3H), 0.81 (t, 3H) 13C NMR (CDC13):…
A:
Q: Write the nuclear equations for the following radioactive processes: Boron-12 (electron capture and…
A:
Q: Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab,…
A: Since, The standard enthalpy of the formation is defined as change of enthalpy of 1 mol of the…
Q: Predict the major product of the following reaction. Enter the InChl code of the structure. + OCH 3
A:
Q: Piperidine is a base found in small amounts in black pepper. What is the pH of 315 mL of an aqueous…
A: The number of moles of any substance is defined as the ratio of the given mass of that substance to…
Q: For the following reaction, 17.1 grams of chlorine gas are allowed to react with 54.4 grams of…
A:
Q: O OCH(CH3)2 + NH3 a. Draw the structure of the tetrahedral intermediate INITIALLY-FORMED in the…
A: The mechanism of given reaction can be explained as:
Q: D July ÖH Product 1 Corresponding Mechanism:
A:
Q: The best Lewis structure for the nitrite ion (NO₂) is Oa Ob Oc Od e _6=o=N] _|o=o_N X [N=O=Ö]¯…
A: Lewis Structure : A Lewis structure is a very simplified representation of the valance shell…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- What are the uses of Laboratory Apparatus below: 1. Aspirator or Pipette Bulb 2. Evaporating Dish 3. Mortar and Pestle 4. Graduated Pipette 5. Acid Burette 6. Spatula 7. Stirring RodConsider a manometer (a barometer-like device used for measuring pressure) constructed using ethyl alcohol (ρ =0.789 g/mL). What would be the column height if the pressure is 24.5 mm Hg? The density of Hg is 13.56 g/mL) h = ____ cmCalculate the volume of the flask using the mass of water which filled the flask and the density of water at the measured temperature. In addition to the volume of the flask, you will need to add 3.0 mL to account for the volume of the tube that leads to the pressure sensor. Table 1 Density of water Temp. (ºC) 20 21 22 23 24 25 26 27 28 29 30 Density (g/mL) 0.9972 0.9970 0.9968 0.9966 0.9964 0.9962 0.9959 0.9957 0.9955 0.9952 0.9949 Mass of flask and stopper: 95.1868 g Mass of flask and stopper filled with water: 230.244 g Temperature of water: 23 C
- Using the equation for the best linear fit to the data (1128.8x + 0.5934), determine the pressure (in atm) in the syringe, if the gas volume was 9.20 mL. (HINT. 1 atm = 760 torr, 1 atm = 760 mm Hg, 1 atm = 101.325 kPa)Covert 797 mm Hg to atm.Calculate the pressure at the base of a mercury column 400 mm high. The mercury density at room temperature is 13.5951 g / cm3.Express the result in column, atmosphere, psi, pascal, and bar units. Calculate the pressure exerted on the drum skin of a diver located at a depth of 20 feet below sea level. Assume that the seawater density is: 1 gr / cm3. Calculate the molar volume of Cu2Te. Express this volume in mol/liter and mol/cm 3 units. ( rm=7.270 g/cm3) Use the ideal gas law and the van der Waals equation to calculate the pressure of ammonia gas at a temperature. T = 300 K the molar volume is V = 24 liters / mol
- Arterial blood pressure is traditionally measured in mm Hg. And the style pressure of 85 mm Hg it’s considered borderline between normal and high blood pressure convert this pressure to kPa units. ( 1atm =760 mm Hg= 101.33 kPa )The data below was collected using the procedure described in your lab notebook: Mass (before reaction): test tube + HCl(aq) + stir bar + capsule 27.000 g Mass (after reaction): test tube + HCl(aq) + stir bar + capsule 25.000 g Volume of water displaced from the squirt bottle 146 mL Temperature of the CO2(g) 298.5 K Pressure (atm) 1.012 atm Using this data answer each of the questions below. Enter your answer to three significant figures and enter only the numerical value in the answer box. Calculate the mass of CO2(g). Calculate the number of moles of CO2(g). Calculate the density of CO2(g) in g/L. Calculate the molar mass of CO2 assuming a temperature of 298.5 K. Calculate the Gas constant, R, in L*atm/K*mol. Calculate the % error of the value of the Gas constant, R.High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to purify chemical substances. The pressures used in this procedure range from around 500 kilopascals (500,000 PaPa) to about 60,000 kPakPa (60,000,000 PaPa). It is often convenient to know the pressure in torr. If an HPLC procedure is running at a pressure of 5.50×108 Pa, what is its running pressure in torr? Express the pressure numerically in torr.
- A pipet delivers 10.9 mLmL , 10.1 mLmL , and 10.9 mLmL in consecutive trials. Find the mean volume of the samples.A previous Chem 100 student carried out the experiment you did by reacting 0.036 g of magnesium. The following data was collected in their lab notebook. The water temperature was 19.8°C. The barometer in the lab read 755.5 mm Hg and 31.97 mL of gas was collected The vapor pressure of water at this temperature is 17.32 mm Hg. Calculate the student’s experimental value of R. Report your answer to four places after the decimal.Recorded barometric pressure: 758.9 mmHg Mass of Aluminum used= .021 g Volume of gas in gas burette after placing into leveling tank= 23 mL temperature in levelling tank: 21 oC Room Temperature: 294.45 K Partial pressure of water vapor: 17.5 mmHg 2 Al (s)+ 6HCl (aq)>>>>2 AlCl3 (aq)+ 3 H2 (g) 1. How to calculate the theoretical yield of H2 gas (mL) based on the mass of Aluminum used. 2. How to calculate the % error of H2 gas.

