Calculate the energy ( in kilojoules ) released when 1.25 g of U- 235 undergoes fission reaction into Ba - 142 and Kr - 92 and neutron/s. The masses are: Ba-142 = 141.92 u %3D Kr - 92 = 91.92 u
Calculate the energy ( in kilojoules ) released when 1.25 g of U- 235 undergoes fission reaction into Ba - 142 and Kr - 92 and neutron/s. The masses are: Ba-142 = 141.92 u %3D Kr - 92 = 91.92 u
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter20: Nuclear Chemistry
Section: Chapter Questions
Problem 20.97QP
Related questions
Question
ANSWER WITH SOLUTION
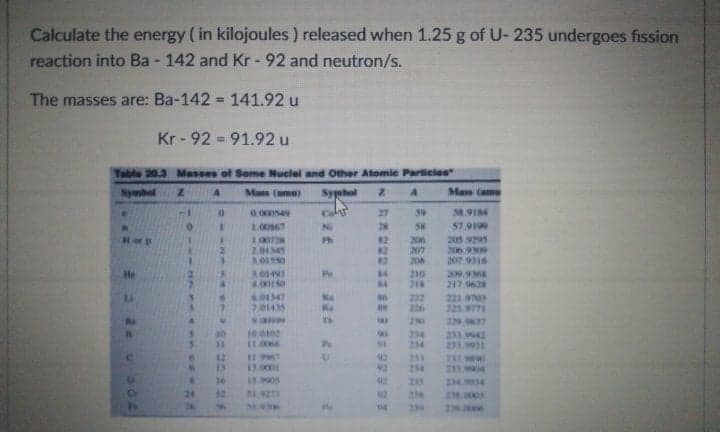
Transcribed Image Text:Calculate the energy ( in kilojoules ) released when 1.25 g of U- 235 undergoes fission
reaction into Ba - 142 and Kr - 92 and neutron/s.
The masses are: Ba-142 = 141.92 u
Kr - 92 = 91.92 u
Table 20.3 Masses of Seme Nuclel and Other Atomic Particies
Nymbel
Mass (m
Symbol
Mass (ame
40
Cols
27
39
38.9184
Ni
28
57.9199
Horp
Ph
82
82
82
206
207
208
205 9295
6066 M
1.0072
2.0145
207.9316
He
3.01493
4.0050
Po
84
210
218
84
217 9628
6.0147
222
226
221.9703
225.9771
7.01435
Re
9.00
Th
230
229 37
234
234
233 9942
233.9931
30
90
1.0066
Po
12
211
13.000
42
234
211.994
16
15.9905
235
234.9034
24
26
52
51.9273
92
235
238.000
239.000
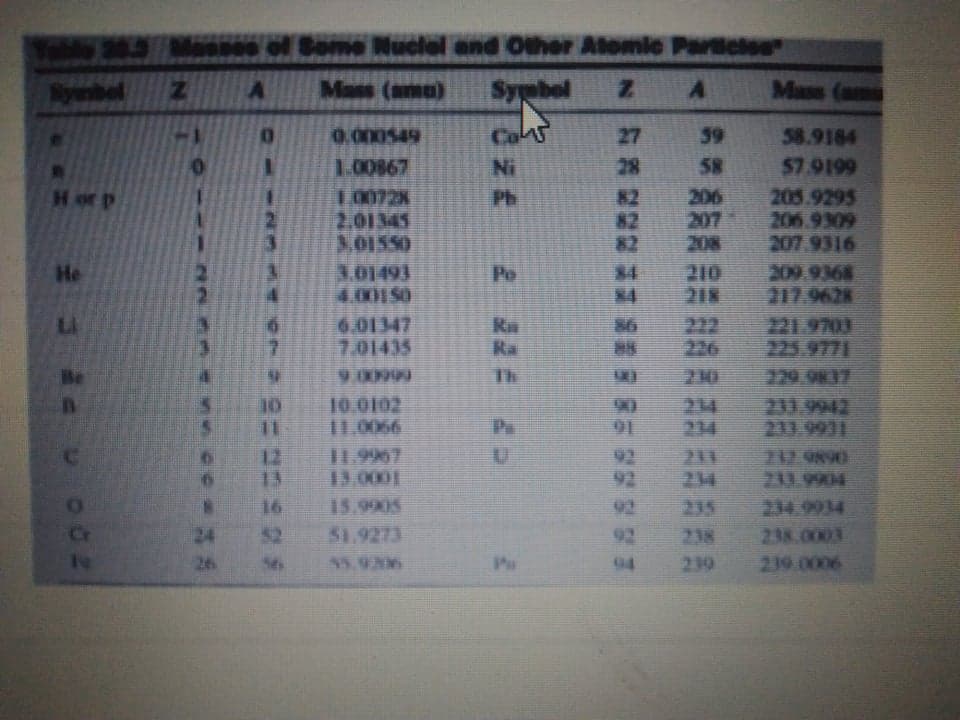
Transcribed Image Text:00 ol Some Nuclel and Other Atomic Paclse
Nyenbel
Mass (amu)
Syebol
Mass (a
0.000549
Co
27
39
58.9184
57.9199
205.9295
206.9309
207.9316
1.00867
Ni
28
58
Hor p
1.0072%
2.01345
3,01550
3.01493
4.001S0
206
207
208
Ph
82
82
82
Po
84
84
210
218
209.9368
217.9628
221.9703
225.9771
729.9817
233.9942
233.9931
He
222
226
LI
6.01347
7.01435
86
Ra
Be
9.00999
Th
210
10.0102.
11.0066
10
90
214
234
Pa
91
12
13
I1.9967
13.000|
92
92
211
214
712 9890
211.9904
234-9934
16
15.9905
92
235
Cr
24
52
51.9273.
92
238
238.0003
26
Pu
94
239
239.0006
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning
