☐ Calculate the energy required to heat 644.0 of silver from 2.1 °C to 14.4 °C. Assume the specific heat capacity of silver under these conditions is 0.235 J.g -1 -1 .K Be sure your answer has the correct number of significant digits. Х x10 μ
☐ Calculate the energy required to heat 644.0 of silver from 2.1 °C to 14.4 °C. Assume the specific heat capacity of silver under these conditions is 0.235 J.g -1 -1 .K Be sure your answer has the correct number of significant digits. Х x10 μ
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
ANSWER IN kJ!!!!!! Careful with sig figs
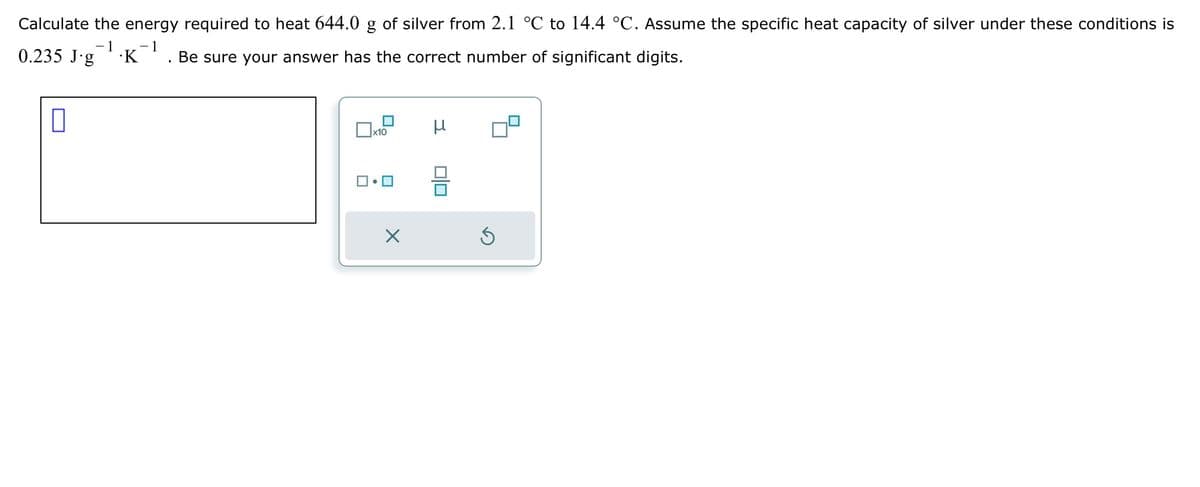
Transcribed Image Text:☐
Calculate the energy required to heat 644.0 of silver from 2.1 °C to 14.4 °C. Assume the specific heat capacity of silver under these conditions is
0.235 J.g
-1 -1
.K
Be sure your answer has the correct number of significant digits.
Х
x10
μ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning