0 A certain half-reaction has a standard reduction potential Ered = +0.60 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 0.50 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. Is there a maximum standard reduction potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. ロ→ロ 0 yes, there is a minimum. E = red Πν no minimum 0 ○ yes, there is a maximum. E = red Ον By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the anode of this cell. ☐ Note: write the half reaction as it would actually occur at the anode. no maximum ☑
0 A certain half-reaction has a standard reduction potential Ered = +0.60 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 0.50 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. Is there a maximum standard reduction potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. ロ→ロ 0 yes, there is a minimum. E = red Πν no minimum 0 ○ yes, there is a maximum. E = red Ον By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the anode of this cell. ☐ Note: write the half reaction as it would actually occur at the anode. no maximum ☑
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 160MP
Related questions
Question
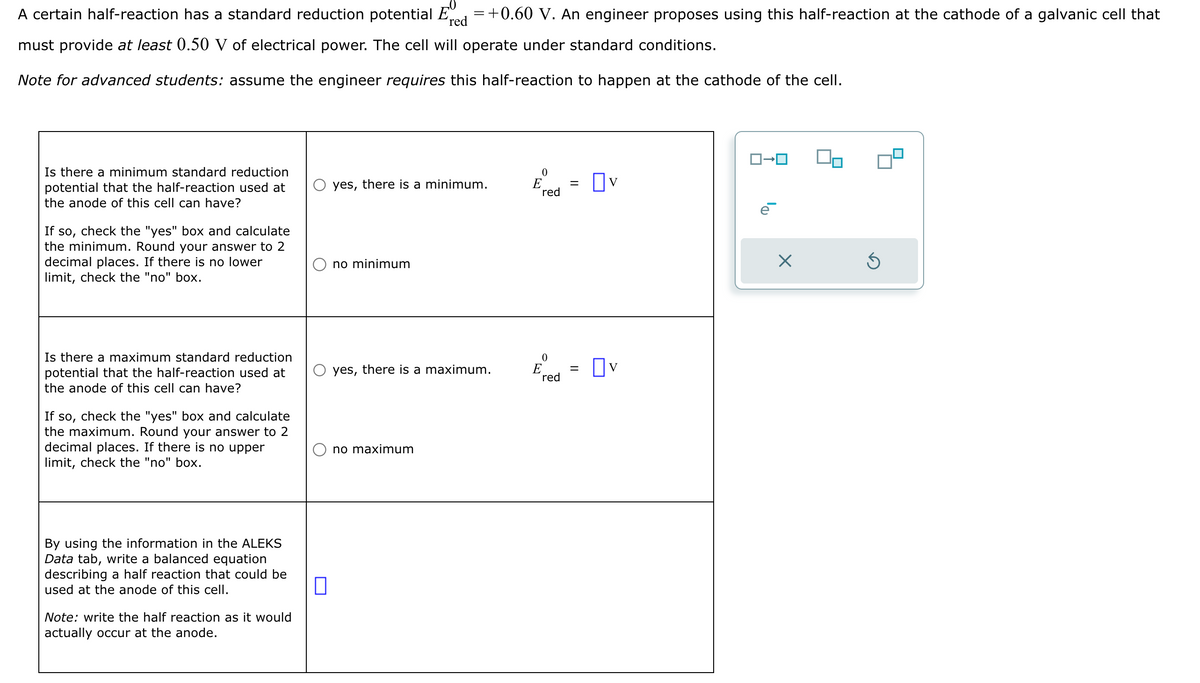
Transcribed Image Text:0
A certain half-reaction has a standard reduction potential Ered = +0.60 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that
must provide at least 0.50 V of electrical power. The cell will operate under standard conditions.
Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell.
Is there a minimum standard reduction
potential that the half-reaction used at
the anode of this cell can have?
If so, check the "yes" box and calculate
the minimum. Round your answer to 2
decimal places. If there is no lower
limit, check the "no" box.
Is there a maximum standard reduction
potential that the half-reaction used at
the anode of this cell can have?
If so, check the "yes" box and calculate
the maximum. Round your answer to 2
decimal places. If there is no upper
limit, check the "no" box.
ロ→ロ
0
yes, there is a minimum.
E
=
red
Πν
no minimum
0
○ yes, there is a maximum.
E
=
red
Ον
By using the information in the ALEKS
Data tab, write a balanced equation
describing a half reaction that could be
used at the anode of this cell.
☐
Note: write the half reaction as it would
actually occur at the anode.
no maximum
☑
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning