Calculate the equilibrium constant for the following reaction at 318 °C: AH°i (kJ mol-1): S° (J mol-1 K-1): N2 (g) + 3 X2 (g)= 2 NX3 (g) 0.0 192 0.0 -43 210 172 -) 2.10 × 10'7 B) 4.28 × 10-18 C) 1.04 D) 0.960 E) 132
Organic Chemistry of Metabolic Pathways
Metabolic pathways allude to the arrangement of chemical catalyzed reactions that lead to the transformation of a substance into the final product. Metabolic pathways incorporate a progression of reaction where the substrate is changed continuously and the transitional metabolites are persistently recovered.
Glucogenesis
Glucogenesis is a metabolic pathway in which glucose is produced from carbon substrates that are not carbohydrates. This process is observed in plants, animals, fungi, bacteria and other micro organisms. The general definition for glucogenesis or gluconeogenesis is as follows,
Answer:B
Please explain steps
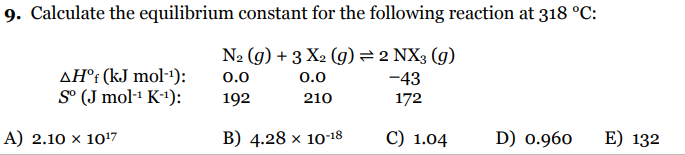
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









