a) Calculate AG° and K, for the following equilibrium reaction at 25°C. The AG°, values are: Cl.(g),0; PCI:(g), -286 kJ/mol; and PCIs(g), -325 kJ/mol. PCI:(g) PCI:(g) + Cl:(g) b) Calculate AG for the reaction if the partial pressures of the initial mixture are: PPCIS = 0.0029 atm, PpCla = 0.27 atm, and Pch = 0.40 atm.
a) Calculate AG° and K, for the following equilibrium reaction at 25°C. The AG°, values are: Cl.(g),0; PCI:(g), -286 kJ/mol; and PCIs(g), -325 kJ/mol. PCI:(g) PCI:(g) + Cl:(g) b) Calculate AG for the reaction if the partial pressures of the initial mixture are: PPCIS = 0.0029 atm, PpCla = 0.27 atm, and Pch = 0.40 atm.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 58QAP: Calculate K at 25°C for each of the reactions referred to in Question 32. Assume smallest...
Related questions
Question
100%
PLEAse show me a complete solution with corresponding units if applicable.
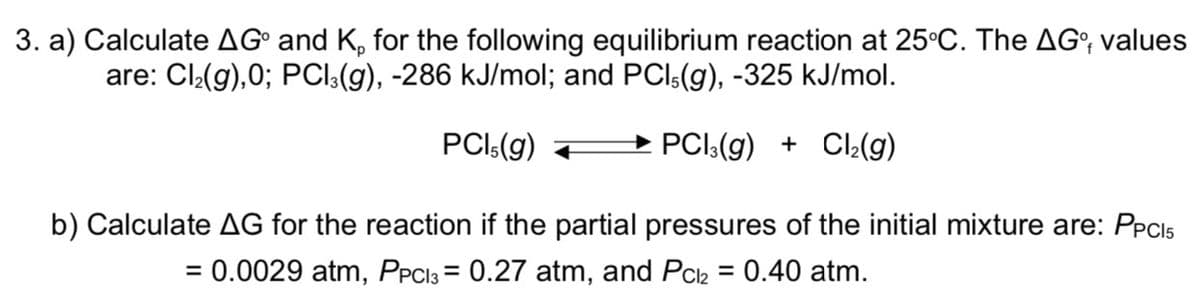
Transcribed Image Text:3. a) Calculate AG° and K, for the following equilibrium reaction at 25°C. The AG°, values
are: Cl:(g),0; PCl:(g), -286 kJ/mol; and PCl:(g), -325 kJ/mol.
PCI:(g)
→ PCI:(g) + Cl:(g)
b) Calculate AG for the reaction if the partial pressures of the initial mixture are: PPCI5
= 0.0029 atm, PPC13 = 0.27 atm, and Pch = 0.40 atm.
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning