Calculate the formal charge of carbon and oxygen in the 2 compounds and compare the polarity of each based from the calculated formal charges H. H H. C H. H 0: H. А. O A is more polar B is more polar O both of them are non-polar O they are both polar B.
Calculate the formal charge of carbon and oxygen in the 2 compounds and compare the polarity of each based from the calculated formal charges H. H H. C H. H 0: H. А. O A is more polar B is more polar O both of them are non-polar O they are both polar B.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter8: Advanced Theories Of Covalent Bonding
Section: Chapter Questions
Problem 37E: Why are bonding molecular orbitals lower in energy than the parent atomic orbitals?
Related questions
Question
100%
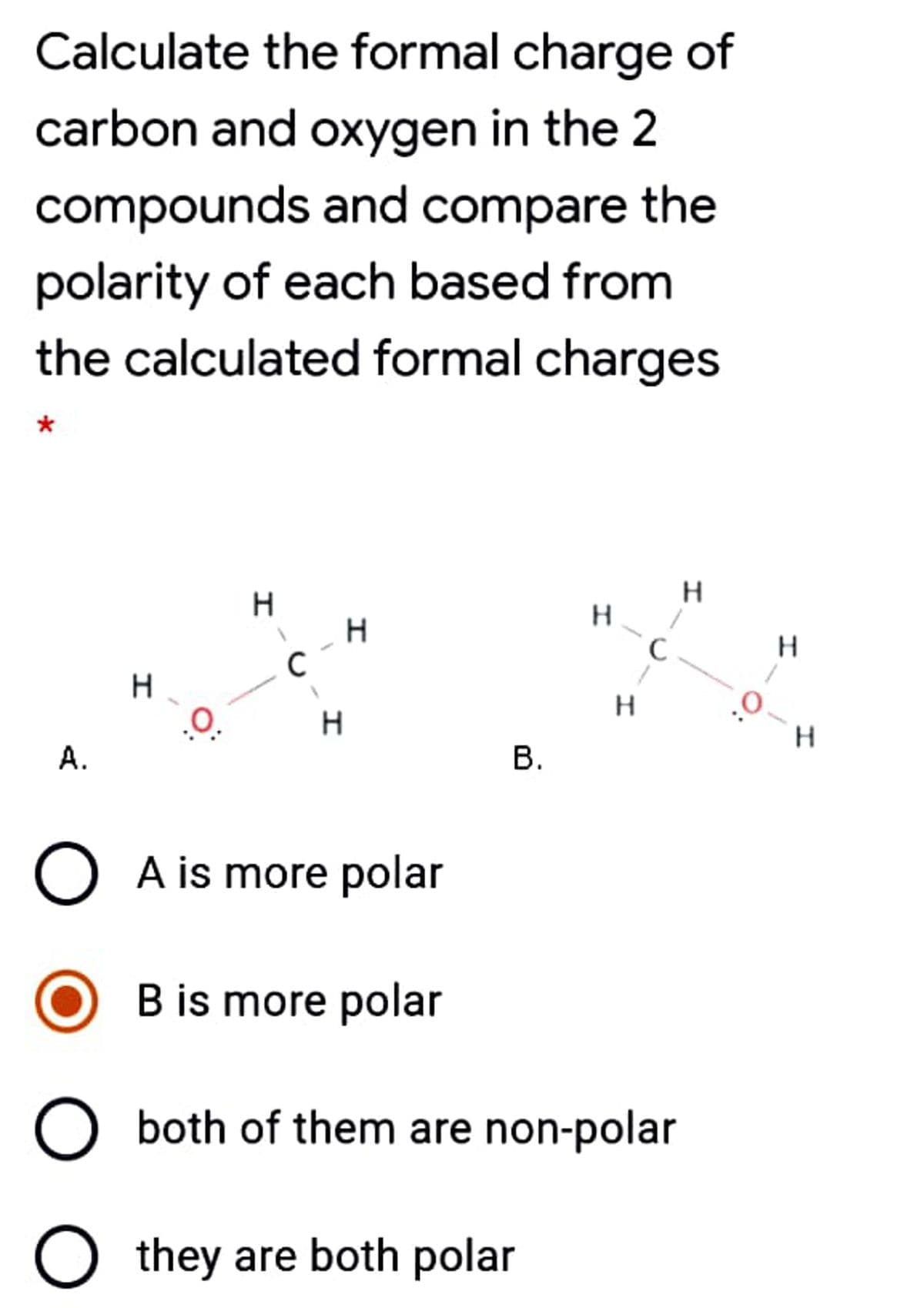
Transcribed Image Text:Calculate the formal charge of
carbon and oxygen in the 2
compounds and compare the
polarity of each based from
the calculated formal charges
*
H.
H.
H.
H.
A.
В.
O A is more polar
B is more polar
both of them are non-polar
they are both polar
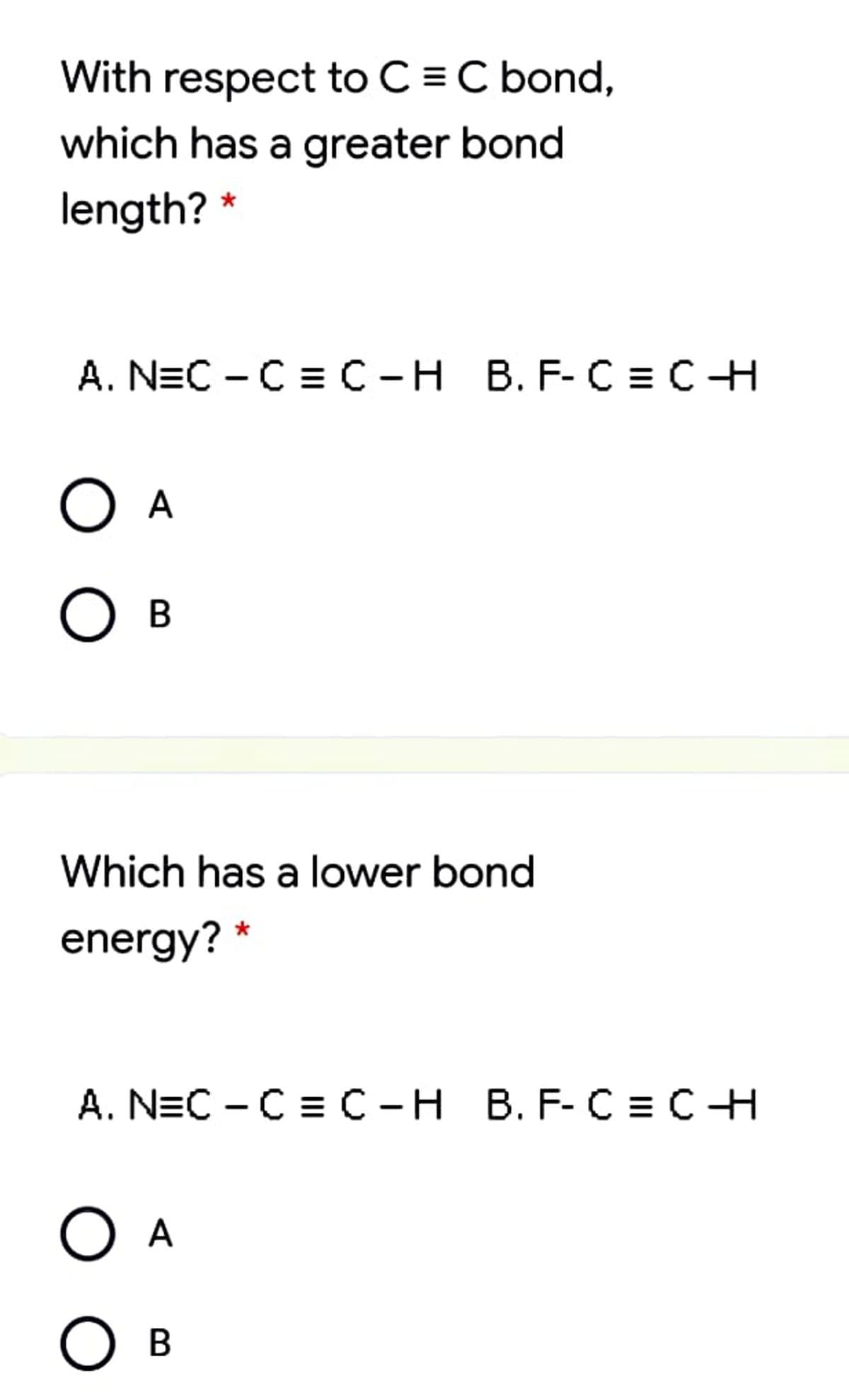
Transcribed Image Text:With respect to C = C bond,
which has a greater bond
length? *
А. NEC - C%3С -Н В. F-С 3Dс Н
O A
Ов
Which has a lower bond
energy? *
A. N=C - C = C-H B. F- C = CH
O A
Ов
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning