Calculate the freezing point of a 10.50 m aqueous solution of sucrose. Freezing point constants can be found in the list of colligative constants. Tf = °C
Calculate the freezing point of a 10.50 m aqueous solution of sucrose. Freezing point constants can be found in the list of colligative constants. Tf = °C
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 88E: The freezing-point depression of a 0.091-m solution of CsCl is 0.320C. The freezing-point depression...
Related questions
Question
How can I solve this question? I need full details and accurate answer.
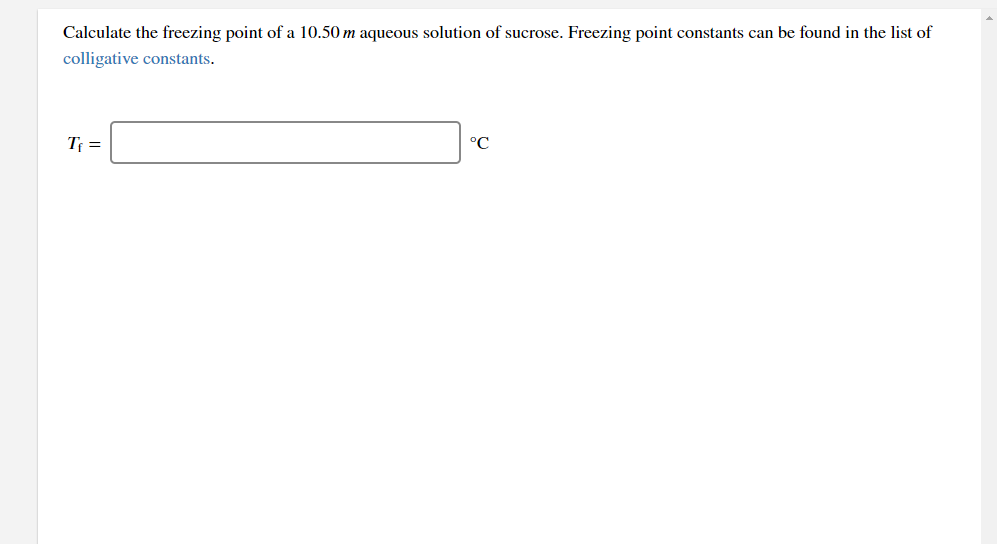
Transcribed Image Text:Calculate the freezing point of a 10.50 m aqueous solution of sucrose. Freezing point constants can be found in the list of
colligative constants.
T =
°C
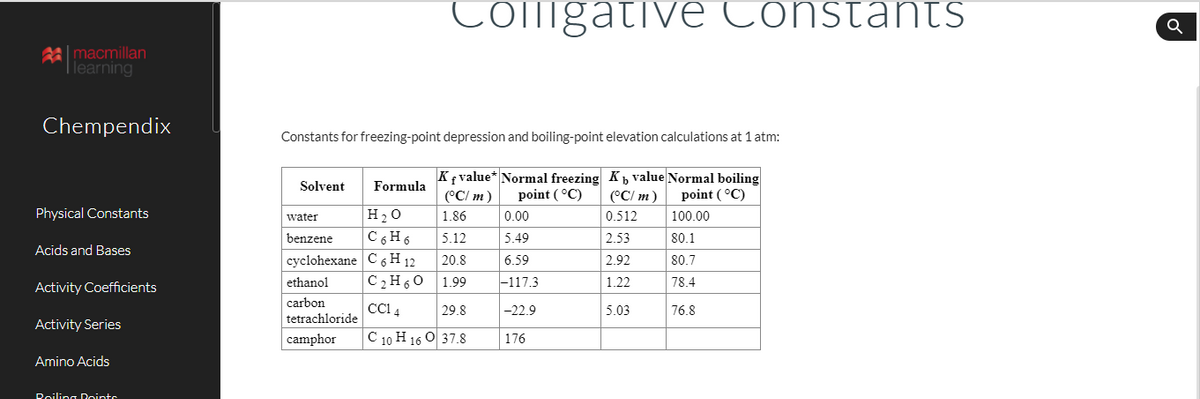
Transcribed Image Text:Coligative Constants
amacmillan
learning
Chempendix
Constants for freezing-point depression and boiling-point elevation calculations at 1 atm:
K{ value* Normal freezing K, value Normal boiling
(°C/ m)
Solvent
Formula
(°C/ m)
point ( °C)
point (°C)
H20
C 6H6
Physical Constants
1.86
0.00
0.512
100.00
water
benzene
5.12
5.49
2.53
80.1
Acids and Bases
сyclohexane C sН 12
C2H60
20.8
6.59
2.92
80.7
Activity Coefficients
ethanol
1.99
|-117.3
1.22
78.4
carbon
CCI 4
29.8
-22.9
5.03
76.8
tetrachloride
Activity Series
camphor
C 10 H 16 O 37.8
176
Amino Acids
Roiling Dointc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax