General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.28QP: If one mole of the following compounds were each placed into separate beakers containing the same...
Related questions
Question
![A cvg.cengagenow.com
Netf
OWLV2 | Online teaching and learning resource from Cengage Learning
[Review Topics]
[References]
Use the References to access important values if needed for this question.
Calculate the hydronium ion concentration in an aqueous solution of 0.195 M carbonic acid, H,CO3 (aq).
[H,O*]=
M.
Submit Answer
Retry Entire Group
8 more group attempts remaining
Visited
Email
Cengage Learning | Cengage Technical Support
MacBook Air
80
888
DD
F3
F4
F5
F6
F7
F8
F9
F10
24
%
&](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F2b4165bd-b363-4d3f-855a-6d431dddeec9%2Fd0de75c3-c37d-48f1-a2ce-fb1a08299845%2Fuousfl9_processed.jpeg&w=3840&q=75)
Transcribed Image Text:A cvg.cengagenow.com
Netf
OWLV2 | Online teaching and learning resource from Cengage Learning
[Review Topics]
[References]
Use the References to access important values if needed for this question.
Calculate the hydronium ion concentration in an aqueous solution of 0.195 M carbonic acid, H,CO3 (aq).
[H,O*]=
M.
Submit Answer
Retry Entire Group
8 more group attempts remaining
Visited
Email
Cengage Learning | Cengage Technical Support
MacBook Air
80
888
DD
F3
F4
F5
F6
F7
F8
F9
F10
24
%
&
![A cvg.cengagenow.com
Netflix
OWLV2 | Online teaching and learning resource from Cengage Learning
[Review Topics)
[References]
Use the References to access important values if needed for this question.
Calculate the concentration of HCO, in an aqueous solution of 0.0105 M carbonic acid, H,CO3 (aq).
[HCO;] =|
М.
Submit Answer
Retry Entire Group
8 more group attempts remaining
Visited
(Previous
Email Instruc
Cengage Learning | Cengage Technical Support
MacBook Air
80
DII
DD
F3
F4
F5
F6
F7
FB
F9
F10
F11
24
&
4.
5
7
6.
* CO](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F2b4165bd-b363-4d3f-855a-6d431dddeec9%2Fd0de75c3-c37d-48f1-a2ce-fb1a08299845%2Fiapnlp_processed.jpeg&w=3840&q=75)
Transcribed Image Text:A cvg.cengagenow.com
Netflix
OWLV2 | Online teaching and learning resource from Cengage Learning
[Review Topics)
[References]
Use the References to access important values if needed for this question.
Calculate the concentration of HCO, in an aqueous solution of 0.0105 M carbonic acid, H,CO3 (aq).
[HCO;] =|
М.
Submit Answer
Retry Entire Group
8 more group attempts remaining
Visited
(Previous
Email Instruc
Cengage Learning | Cengage Technical Support
MacBook Air
80
DII
DD
F3
F4
F5
F6
F7
FB
F9
F10
F11
24
&
4.
5
7
6.
* CO
Expert Solution
Step 1
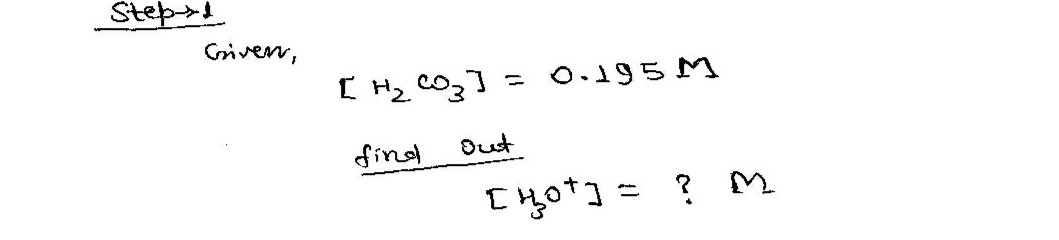
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax