Calculate the mass, in grams, of each sample. a. 1.1 x 10 gold atoms b. 2.82 x 102 helium atoms c. 1.8 x 1023 lead atoms d. 7.9 x 1021 uranium atoms
Calculate the mass, in grams, of each sample. a. 1.1 x 10 gold atoms b. 2.82 x 102 helium atoms c. 1.8 x 1023 lead atoms d. 7.9 x 1021 uranium atoms
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter4: The Structure Of The Atom
Section: Chapter Questions
Problem 11STP
Related questions
Question
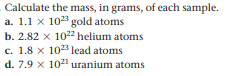
Transcribed Image Text:Calculate the mass, in grams, of each sample.
a. 1.1 x 10 gold atoms
b. 2.82 x 102 helium atoms
c. 1.8 x 1023 lead atoms
d. 7.9 x 1021 uranium atoms
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

