Calculate the mass of copper sulfate hydrate placed in crucible for a trial 2. Calculate the mass of anhydrous copper sulfate and crucible for each trial 3. Calculate the mass of water driven off each trial 4. Calculate moles of anhydrous copper sulfate in moles of water
Calculate the mass of copper sulfate hydrate placed in crucible for a trial 2. Calculate the mass of anhydrous copper sulfate and crucible for each trial 3. Calculate the mass of water driven off each trial 4. Calculate moles of anhydrous copper sulfate in moles of water
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 151AE: A 0.755-g sample of hydrated copper(II) sulfate CuSo4xH2O was heated carefully until it had changed...
Related questions
Question
1.Calculate the mass of copper sulfate hydrate placed in crucible for a trial
2. Calculate the mass of anhydrous copper sulfate and crucible for each trial
3. Calculate the mass of water driven off each trial
4. Calculate moles of anhydrous copper sulfate in moles of water
5. determine the ratio of the moles of water to the moles of copper sulfate for each trial
6.
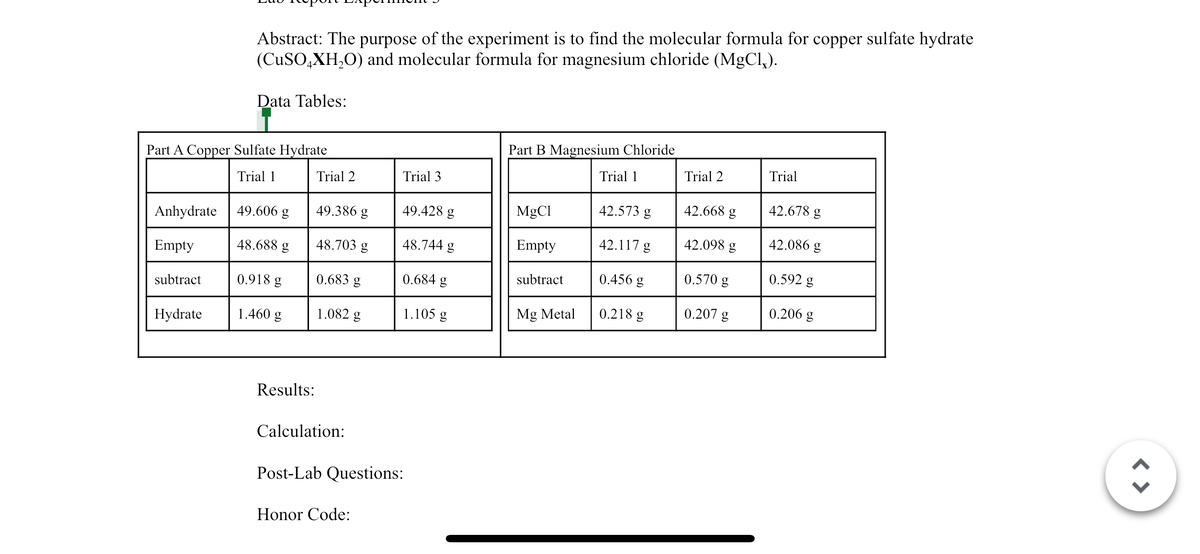
Transcribed Image Text:Anhydrate
Empty
Part A Copper Sulfate Hydrate
Trial 1
subtract
Abstract: The purpose of the experiment is to find the molecular formula for copper sulfate hydrate
(CuSO4XH₂O) and molecular formula for magnesium chloride (MgCl₂).
Hydrate
Data Tables:
49.606 g
48.688 g
0.918 g
1.460 g
Results:
Trial 2
49.386 g
48.703 g
0.683 g
1.082 g
Calculation:
Trial 3
Honor Code:
49.428 g
48.744 g
0.684 g
1.105 g
Post-Lab Questions:
Part B Magnesium Chloride
Trial 1
MgCl
Empty
subtract
Mg Metal
42.573 g
42.117 g
0.456 g
0.218 g
Trial 2
42.668 g
42.098 g
0.570 g
0.207 g
Trial
42.678 g
42.086 g
0.592 g
0.206 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning