Calculate the mass of Mg (g) Mass of watch glass, evaporating dish, and reacted Mg after cooling: Calculate the mass of the product (g) Calculate the mass of Cl (g) Mole calculations Calculate the moles of Mg in the product (mol) Calculate the moles of Cl in the product (mol) Determining the Empirical Formula Which element has the smallest number of moles? Table EF.1: Determining the Empirical Formula 0.28 1.121 Ma 0.841 0.0115 0,0236 Table viewi 63.375 g List view Saved Saved Saved Saved Saved Saved
Calculate the mass of Mg (g) Mass of watch glass, evaporating dish, and reacted Mg after cooling: Calculate the mass of the product (g) Calculate the mass of Cl (g) Mole calculations Calculate the moles of Mg in the product (mol) Calculate the moles of Cl in the product (mol) Determining the Empirical Formula Which element has the smallest number of moles? Table EF.1: Determining the Empirical Formula 0.28 1.121 Ma 0.841 0.0115 0,0236 Table viewi 63.375 g List view Saved Saved Saved Saved Saved Saved
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 41QAP: Which of the following statements is(are) true? l type='a'> A balanced equation relates the numbers...
Related questions
Question
100%
Hi would someone please be able to double check my work thank you ??
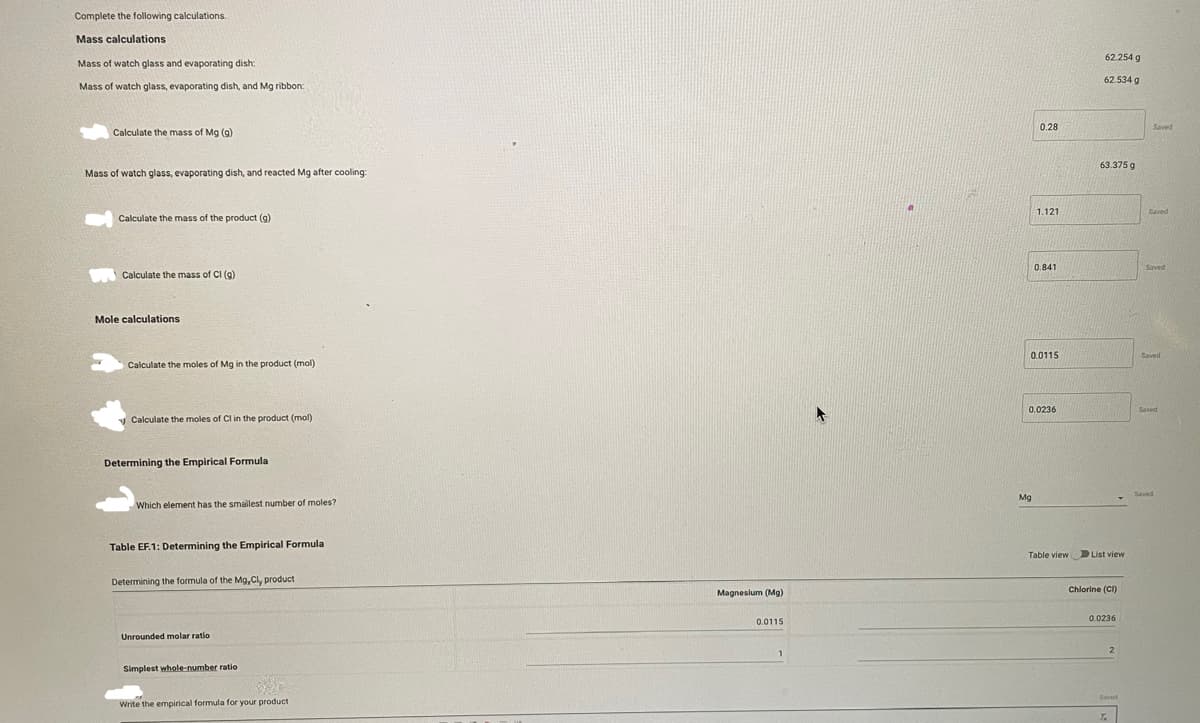
Transcribed Image Text:Complete the following calculations.
Mass calculations
Mass of watch glass and evaporating dish:
Mass of watch glass, evaporating dish, and Mg ribbon:
Calculate the mass of Mg (g)
Mass of watch glass, evaporating dish, and reacted Mg after cooling:
Calculate the mass of the product (g)
Calculate the mass of Cl (g)
Mole calculations
Calculate the moles of Mg in the product (mol)
Calculate the moles of Cl in the product (mol)
Determining the Empirical Formula
Which element has the smallest number of moles?
Table EF.1: Determining the Empirical Formula
Determining the formula of the Mg, Cly product
Unrounded molar ratio
Simplest whole-number ratio
Write the empirical formula for your product
Magnesium (Mg)
0.0115
1
(2014-04
0.28
1.121
0.841
0.0115
0.0236
62.254 g
62.534 g
63.375 g
Table view List view
Chlorine (CI)
0.0236
2
Saved
T.
Saved
Saved
Saved
Saved
Saved
Saved
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning