Calculate the mass percent of CI in (C2CI,F2), a CFC refrigerant: At.Wt. C = 12,F= 19 , Cl = 35.45 (g/mol) %3D O 40.2% O 18.6 % O11.8 % O 80.4% O 69.60 %
Calculate the mass percent of CI in (C2CI,F2), a CFC refrigerant: At.Wt. C = 12,F= 19 , Cl = 35.45 (g/mol) %3D O 40.2% O 18.6 % O11.8 % O 80.4% O 69.60 %
Algebra: Structure And Method, Book 1
(REV)00th Edition
ISBN:9780395977224
Author:Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Chapter12: Quadratic Functions
Section12.6: Solving Problems Involving Quadratic Equations
Problem 1.4E
Related questions
Question
Quickly please
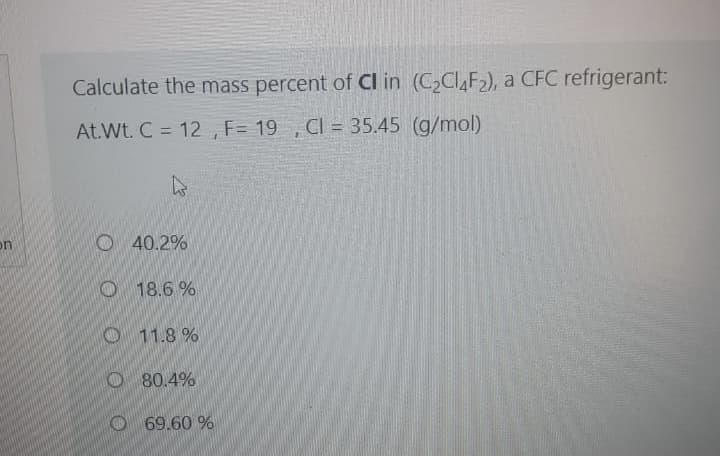
Transcribed Image Text:Calculate the mass percent of Cl in (C2CI,F2), a CFC refrigerant:
At.Wt. C = 12 , F= 19 , CI = 35.45 (g/mol)
%3D
O 40.2%
on
O 18.6 %
O11.8 %
O80.4%
O 69.60 %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,