Calculate the maximum pressure reached in cycle (in kPa) Calculate the maximum temperature reached in cycle (in K) Calculate the volume after completion of isothermal expansion process (in dm')
Calculate the maximum pressure reached in cycle (in kPa) Calculate the maximum temperature reached in cycle (in K) Calculate the volume after completion of isothermal expansion process (in dm')
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 98CP: In a diesel engine, the fuel is ignited without a spark plug. Instead, air in a cylinder is...
Related questions
Question
hellllooo :)
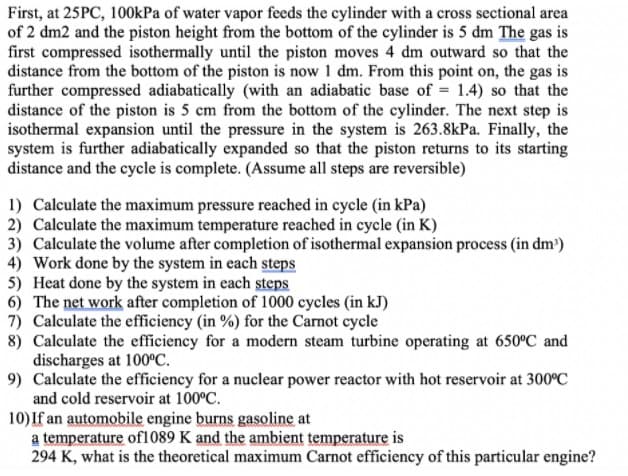
Transcribed Image Text:First, at 25PC, 100kPa of water vapor feeds the cylinder with a cross sectional area
of 2 dm2 and the piston height from the bottom of the cylinder is 5 dm The gas is
first compressed isothermally until the piston moves 4 dm outward so that the
distance from the bottom of the piston is now 1 dm. From this point on, the gas is
further compressed adiabatically (with an adiabatic base of = 1.4) so that the
distance of the piston is 5 cm from the bottom of the cylinder. The next step is
isothermal expansion until the pressure in the system is 263.8kPa. Finally, the
system is further adiabatically expanded so that the piston returns to its starting
distance and the cycle is complete. (Assume all steps are reversible)
1) Calculate the maximum pressure reached in cycle (in kPa)
2) Calculate the maximum temperature reached in cycle (in K)
3) Calculate the volume after completion of isothermal expansion process (in dm)
4) Work done by the system in each steps
5) Heat done by the system in each steps
6) The net work after completion of 1000 cycles (in kJ)
7) Calculate the efficiency (in %) for the Carnot cycle
8) Calculate the efficiency for a modern steam turbine operating at 650°C and
discharges at 100°C.
9) Calculate the efficiency for a nuclear power reactor with hot reservoir at 300°C
and cold reservoir at 100°C.
10) If an automobile engine burns gasoline at
a temperature of1089 K and the ambient temperature is
294 K, what is the theoretical maximum Carnot efficiency of this particular engine?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning