Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.3QAP
Related questions
Question
Transcribed Image Text:723), Topic: Unit 6:..
McGraw-Hill Education Campus
work
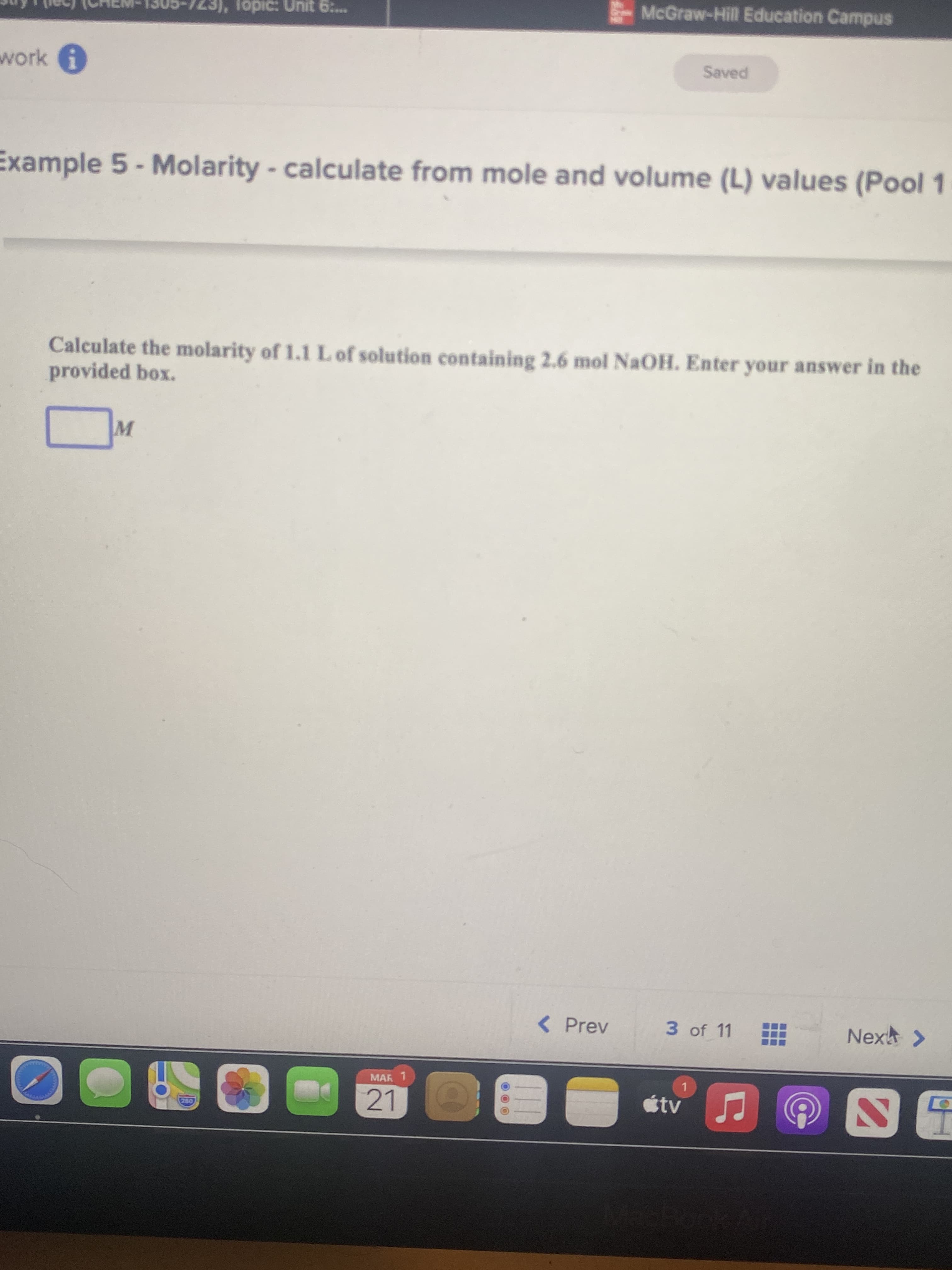
Example 5 - Molarity - calculate from mole and volume (L) values (Pool 1
Calculate the molarity of 1.1LO solution containing 2.6 mol NaOH. Enter your answer in the
provided box.
< Prev
3 of 11
Nex >
MAR 1
1.
21
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
