Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 108GQ
Related questions
Question
100%
Calculate the moles please
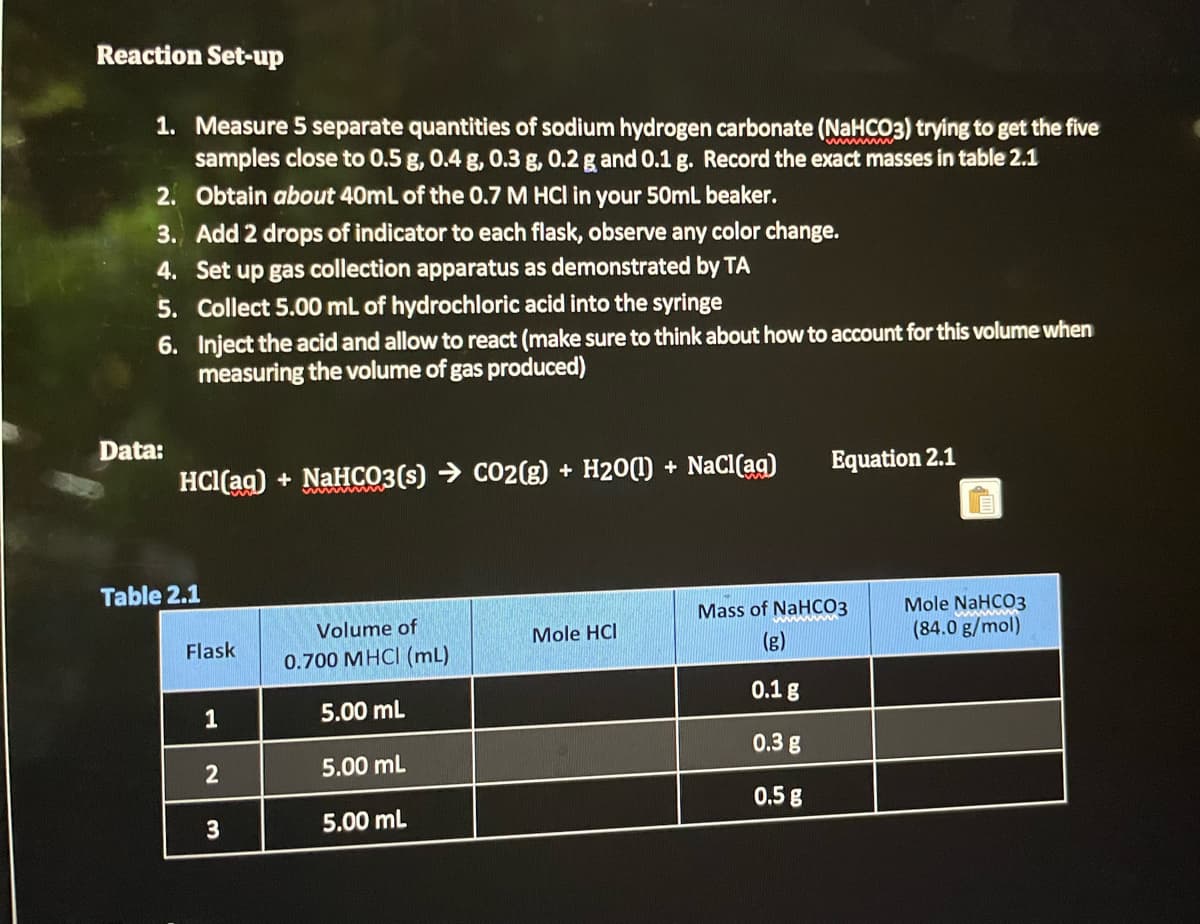
Transcribed Image Text:Reaction Set-up
1. Measure 5 separate quantities of sodium hydrogen carbonate (NaHCO3) trying to get the five
samples close to 0.5 g, 0.4 g, 0.3 g, 0.2 g and 0.1 g. Record the exact masses in table 2.1
2. Obtain about 40mL of the 0.7 M HCl in your 50mL beaker.
3. Add 2 drops of indicator to each flask, observe any color change.
Set up gas collection apparatus as demonstrated by TA
4.
5. Collect 5.00 mL of hydrochloric acid into the syringe
6.
Data:
Inject the acid and allow to react (make sure to think about how to account for this volume when
measuring the volume of gas produced)
HCl(aq) + NaHCO3(s) → CO2(g) + H2O(l) + NaCl(aq)
Table 2.1
Flask
1
2
3
Volume of
0.700 MHCI (mL)
5.00 mL
5.00 mL
5.00 mL
Mole HCI
Equation 2.1
Mass of NaHCO3
(g)
0.1 g
0.3 g
0.5 g
Mole NaHCO3
(84.0 g/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning