Calculate the osmotic pressure of a magnesium citrate laxative solution containing 28.5 g of magnesium citrate in 234 mL of solution at 37 °C (approximate body temperature). Assume complete dissociation of the ionic compound.
Calculate the osmotic pressure of a magnesium citrate laxative solution containing 28.5 g of magnesium citrate in 234 mL of solution at 37 °C (approximate body temperature). Assume complete dissociation of the ionic compound.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 68QAP: Consider two solutions at a certain temperature. Solution X has a nonelectrolyte as a solute and an...
Related questions
Question
100%
Calculate the osmotic pressure of a magnesium citrate laxative solution containing 28.5 g of magnesium citrate in 234 mL of solution at 37 deg * C (approximate body temperature). Assume complete dissociation of the lonic compound.
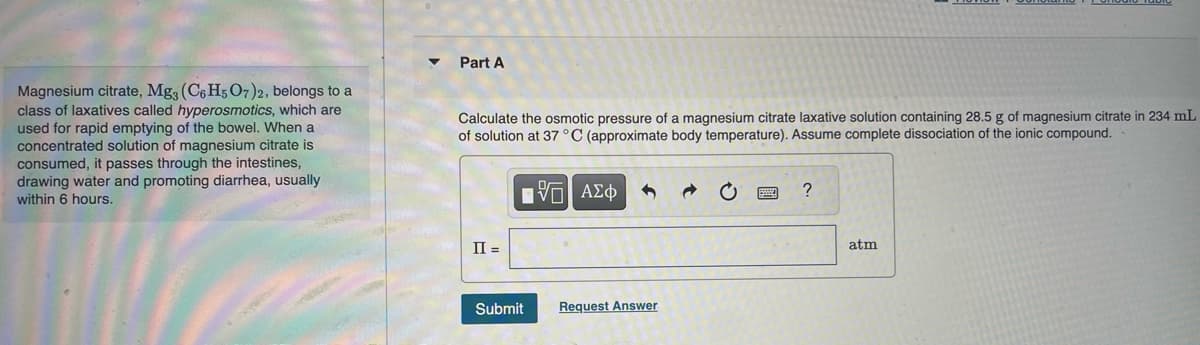
Transcribed Image Text:Magnesium citrate, Mg3 (C6H5 O7)2, belongs to a
class of laxatives called hyperosmotics, which are
used for rapid emptying of the bowel. When a
concentrated solution of magnesium citrate is
consumed, it passes through the intestines,
drawing water and promoting diarrhea, usually
within 6 hours.
xes
▼
Part A
Calculate the osmotic pressure of a magnesium citrate laxative solution containing 28.5 g of magnesium citrate in 234 mL
of solution at 37 °C (approximate body temperature). Assume complete dissociation of the ionic compound.
FE ΑΣΦ
II =
Submit Request Answer
?
atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax