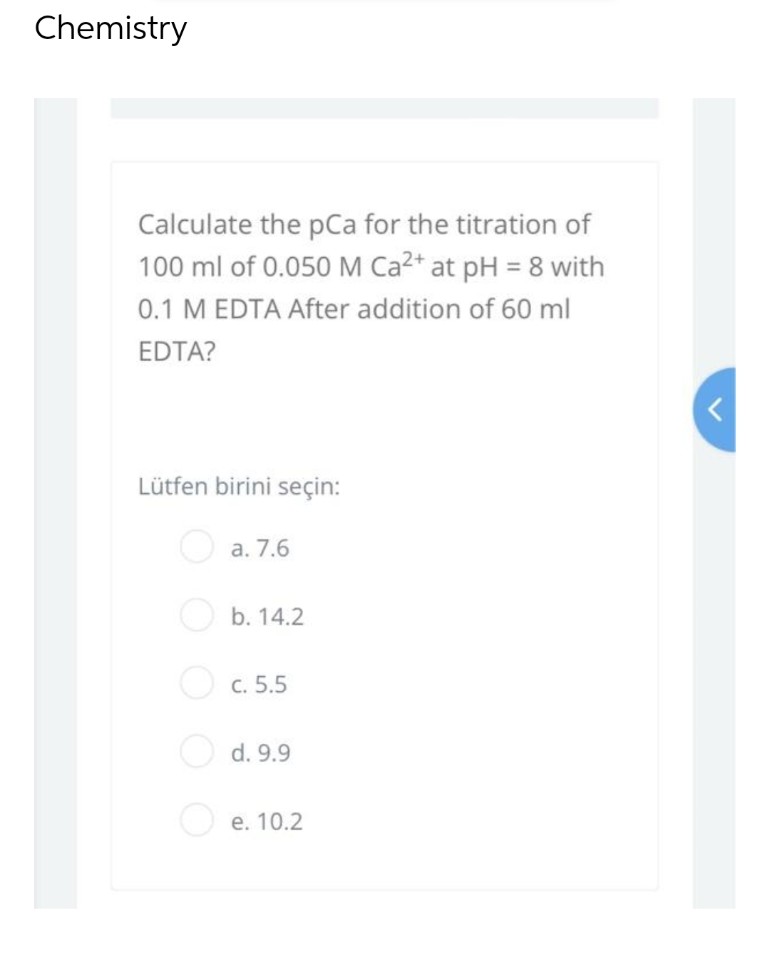
Calculate the pCa for the titration of 100 ml of 0.050 M Ca²+ at pH = 8 with 0.1 M EDTA After addition of 60 ml EDTA? Lütfen birini seçin: a. 7.6 b. 14.2 c. 5.5 d. 9.9 e. 10.2
Q: That is the energy of crystallization for potassium fluoride, given that, for potassium: 89.2…
A: Given data set: Vaporization energy ∆Hvap = 89.2 kJ/mol Ionization energy I.E = -328 kJ/mol Electron…
Q: If 1.1g of 100.0C steam begins to condense on a container of liquid water at 25.0C and they…
A:
Q: IR Spectrum (liquid film) 4000 100 80 60 % of base peak 29 3000 57 74 40 80 13C NMR Spectrum (50.0…
A: Number of signals in 1H NMR = number of sets of protons in different electronic environment
Q: e peptide amide bonds exist either as s-cis or s-trans conformation? conformations have the large…
A: Cis configuration means two bulky or lighter groups are present on same side of double bond.
Q: The absorbance of 1.48 x 105 M methyl red solution, prepared in 0.1 M HCI, was measured using a 1.0…
A:
Q: What is the density of a substances in mg/L, if a sample with a volume of 1.91cm has a mass of…
A: I have given a detailed solution to the question below:
Q: An element with the electron configuration [noble gas] ns2 (n-1)d10 np4 has [number] valence…
A: We know that, Number of valence electrons= number of electrons present in valence shell
Q: (SHOW CALCULATIONS) Calculate the enthalpy of the following reaction using the bond energies given.…
A:
Q: Drag each tile to the correct location. Match each process to where it occurs in the carbon cycle.…
A: There are many cycles on the earth that lead to the balance of the natural system. Each cycle has…
Q: What intermolecular forces would exist between ethanol, an alcohol which has the molecular formula…
A: Given-> Molecules are-> Ethanol => C2H6O Sulphur hexa chloride = SCl6
Q: During kinetic resolution, the reaction of a racemic mixture of an N-acetylated amino acid with a…
A: A racemic mixture, also known as a racemate, is a mixture of two enantiomers, or compounds with…
Q: Give the molecular formula of the molecule and the name of the molecule 2. . List and identify all…
A: A organic molecule is given .we have to find its formula ,IUPAC nomenclature and identify the…
Q: A 1.00 L flask initially contains 4.40 mol of HI at a certain temperature. The K at this temperature…
A:
Q: 34. ................annot be heating and reshaped is. OA. Thermoset. OB. Thermoplastic. 35. The…
A:
Q: نقطة واحدة نقطة واحدة نقطة واحدة Methane molecule CH4 - 26 .hybridization is A - Sp³ B-Sp¹ C-Sp² A B…
A:
Q: Suppose you are measuring the mass of a solid sample on a balance using a weigh boat. You record the…
A: given, The mass of weigh boat = 3.451 g. Mass of weigh boat and sample =…
Q: Q7. What is the correct Lewis structure for the molecule in the box, including the formal charge(s),…
A:
Q: Chemistry What is the reason why peptide amide bonds can be in the s-cis or s-trans conformation?
A: Cis configuration means two bulky or lighter groups are present on same side of double bond.
Q: General formula of aromatic - 22/ .compounds are A - CnH2n. B - CnHn+2. O C - CnH2n-2. O
A: Aromatic compounds are composed of a planar ring and pi-electron cloud is delocalized above and…
Q: If a tablet of methotrexate contains 3.75 mg, how many micrograms of the methotrexate are in five…
A: Given that, mass of 1 tablet= 3.75 mg then,
Q: 35. The polymer is if heated too much, they burn is. A. Thermoset. B. Thermoplastic.
A: Thermo plastics soften on heating whereas thermosetting plastics do not soften on heating.…
Q: 2.4 mol of PCI,(g) are injected into a 2.0 L container and the following equilibrium becomes…
A:
Q: 11.Residual EDTA titrations are employed in the analysis of Bi and ________ containing compounds.…
A: Complexometric titration : A titration based on the formation of a cordination complex is known as…
Q: .The unit of specific gravity is - 51 A - g/cm2. O B - Without unite. O C-g/cm3. O
A: Specific gravity is the ratio between the density of substance to the density of reference…
Q: buffer solution contains 0.365 M NaHCO3 and 0.305 M K2CO3. If 0.0385 moles of potassium hydroxide…
A: Given-> Molarity of NaHCO3 = 0.365 M Molarity of K2CO3 = 0.305 M Moles of KOH = 0.0385 mole…
Q: What is the major product of the reaction shown below? A) butane B) 1-butene H₂ C) 1-butyne Pt, heat…
A: Addition of hydrogen to a carbon-carbon double bond is known as hydrogenation. With addition of…
Q: Steam is compressed reversibly to liquid water at the boiling point 100°C. The heat of vaporization…
A: Solutions Find W per mole and Q per mole and each of the thermodynamic quantities...
Q: 2 Al +31,-2 All, 2 NEXT Assume the reaction starts with 4.20 g Al (26.98 g/mol) and 17.40 g iodine,…
A:
Q: 50-The unit of density is. OA-g/cm2. OB - Km/cm. OC-g/cm3. 51-The unit of specific gravity is.…
A: Density = Mass / volume
Q: نقطة واحدة نقطة واحدة نقطة واحدة १:.१ Most monomers are organic .12 materials, atoms are joined in…
A: Given in following question some polymer releated subjective question choose a correct answer
Q: 11. The earliest synthetic polymer was developed in 1906, called Bakelite *** O A. Polyethene. OB.…
A:
Q: A OH 01 NH₂ B OH NH₂ A) Compound A B) Compound B C) Compound C D) Compound D
A: Alkenes Hydrocarbons which contain carbon-carbon double bonds. General formula is CnH2n for…
Q: A 1.00 L flask initially contains 4.40 mol of HI at a certain temperature. The K at this temperature…
A:
Q: What is the product of the reaction below? Xo ค้ำชู LOH + назад DCC
A:
Q: 12) The most abundant porphyrins in nature are found in hemoglobin and the chlorophylls. The parent…
A:
Q: 17. ********** melted. OA. Thermoplastics *********** are cured into permanent shape is Cannot be…
A:
Q: To convert 20 g of ice at -5oC to 120oC to steam you need _______ cal of energy?
A:
Q: Identify the product of the following reaction (Hint: no acidic workup was needed to forge A A
A: Organic reactions are those in which organic reactant react to form organic products.
Q: Given the transition state diagram(s) below, identify the mechanism(s) of catalysis. Mg2+ H-N(CH₂)…
A: In the given catalysis reaction, there is no metal ion involved which initiates or catalyses the…
Q: In the Gabriel synthesis of primary amines, N-potassiophthalimide is used as a source of the…
A: Here we have to write products formed in step-1 and step-2 in the following given Gabriel synthesis.…
Q: Chemistry Which of the following describe alpha? (choose one or more) The level of significance The…
A: Alpha is a Greek alphabet. Here we have to choose the correct option which describes the symbol…
Q: Determine the entropy of * 2H2(g) + O2(g) → 2H₂O(1) positive negative
A:
Q: Salts of carbonate ion behave as a weak base, undergoing hydrolysis in water according to the…
A:
Q: The important steps in solid phase peptide synthesis are? A) Coupling and hydrolysis B) Hydrolysis…
A: Solid-phase peptide synthesis (SPPS) involves the successive addition of protected amino acid and…
Q: which naturally.... .10 .occurring polymer is A. Polyethene. O B. PVC. O C. Wool. O The earliest…
A:
Q: Predict the major and minor product(s) for the following reactions: CI NaOH t-BuOK H₂O heat J.. CI…
A:
Q: Use the following atomic masses (in g/mol): As = 74.92; O = 16; K = 39.1; Cr = 52; Na = 23; Cl =…
A:
Q: According to valence bond theory, the triple bond in ethyne (acetylene, C₂H₂) consists of O three…
A: Sigma bond is formed by head to head overlap while pi-bond is formed by sideways overlapping. Sigma…
Q: What would be the product if D-galactose is react with 1. NH2OH then (CH3CO)2O, NaOCOCH3 then…
A: Carbonyl compound of aldehyde react with NH2OH to give C=N-OH HNO3 is strong oxidizing agent it can…
Q: (SHOW WORK) Use the condensed electron configuration to write an equation for the formation of the…
A: Here, we have to write an equation for the formation of N3- ion using the condensed electron…
Step by step
Solved in 3 steps

- The potentiometric titration data of 2,422 mmol chloride ion with 0.1000 M AgNO3 are as follows. What should the x and y axis values be to find the turning point using the first derivative curve? AgNO3 volume (mL) Potential (Volts) 5.0 0.062 15.0 0.085 20.0 0.107 22.0 0.123 23.0 0.138 23.50 0.146 23.80 0.161 24.00 0.174 24.10 0.183 24.20 0.194 24.30 0.233100 mL of mineral water containing Mg2+ and Ca2+ is taken and titrated with 86.65 mL of 0.06120 M EDTA. NH4F was added to the second 100 mL portion taken from the mineral water to mask the magnesium in the sample as MgF2, and when this sample was titrated with the same EDTA solution, 38.56 mL was spent. Find the concentration of CaCO3 and MgCO3 in the sample in ppm.Calculate the pCa for the titration of 50 ml of 0.02 M Ca2+ at pH = 8 with 0.4 M EDTA after addition of 40 ml EDTA? Ca2+ + Y4- ⇄ CaY2- Kf = 5x1010 a. 9.6 b. 4.9 c. 7.7 d. 10.2 e. 7.6
- Consider the titration of 25.0mL 0.020M of Co(NO3)2 with .0100M EDTA in a solution buffered to pH 10.00. Calculate pCo^2+ at the following volumes of added EDTA. a. 0.0 mL b. 20.0 mL c. 40.0 mL d. 49.0 mL e. 50.0 mL f. 50.1 mL g. 55.0 mL h. 60.0 mLCalculate the pCa for the titration of 50 ml of 0.02 M Ca2+ at pH = 8 with 0.4 M EDTA at the equivalence point Ca2+ + Y4- ⇄ CaY2- Kf = 5x1010 Select one: a. 7 b. 12 c. 10 d. 5 e. 9A 35.0 mL of 1.00 x10–2 M Ca2+ was titrated with 0.0250 M EDTA at a pH of 10 and in the presence of 0.0500 M NH3. The formation constant for Ca2+–EDTA is 4.5 x1010. *αY4– = 0.35 at a pH 10; αCa2+ = 0.969 when the concentration of NH3 is 0.0500 M. Calculate the pCa (20pts) a.) Before equivalence point b.) At equivalence point c.) After equivalence point
- Titration of the I2 produced from 0.2645g of primary standard KIO3 required 45.36mL of sodium thiosulfate. Calculate the concentration of the Na2S2O3 in M. IO3- + 5I- + 6H+ --> 3I2 + 3H2O I2 + 2S2O32- --> 2I- + S4O62- KIO3 mw = 214.001g/molA commercial lab received a batch of industrial wastewater samples for analysis. Johnplans to test water hardness of these unknown samples. Prior to complexometric titration,the titrant, EDTA solution needs to be standardized. A 50.00 mL of 5.67 x10-3 M Ca2+standard solution required average of 38.10 mL of unknown EDTA solution to reach itsend point. Based on titration information recorded, work out the correct molarity of EDTAsolution.A solution containing 60 mL of a 0.025 mol / L metal ion (Mn +) buffer buffered to pH 7.0 was titrated with a 0.05 mol / L EDTA solution. Data: Conditional formation constant (Kf)=10^15. Determine: i) The concentration of the free metal when ½ of the equivalence volume is added. ii) the concentration of free metal in the equivalence volume. iii) the concentration of free metal with an excess of 2 mL of EDTA? (Data - EDTA constants: K1=0.01; K2=2.19.10^-3; K3=6.92.10^-7; K4=5.75.10^-11)
- Which of the following EDTA titration formats can be used when the analyte reacts too slowly with EDTA? Direct titration Back titration Displacement titration Indirect titration 1 only 2 only 2 & 3 3 only 4 onlyYou took 25.00 mL of unknown water solution and titrated it with EDTA. The EDTA solution molarity was 0.01 M, and it took 16.50 mL to reach the end point. With the blank it took only 0.98 mL to reach end point. 15.52 mL of EDTA solution were used to titrate hardness that actually came from the unknown 1.552×10−4 moles of EDTA reacted with hardness-causing ions from the unknown sample 1.552×10−4 moles of hardness-causing ions were present in the unknown sample A) Assuming that the total hardness of water is due to CaCO3, how many grams CaCO3 does it correspond to? B) What is the total hardness of the unknown water in ppm CaCO3?Titration of 50.00 mL of 0.04715 M Na2C2O4 required 39.25 mL of a potassium permanganate solution. 2MnO4- + 5C2O4-2 +16H+ -> 2Mn2+ + 10CO2(g) + 8H2O Calculate the molar concentration of the KMnO4 solution What molecules would interfere with the titrimetric analysis?



