Calculate the %(m/v) of hydrochloric acid using the molar concentration you found. Shows calculations. Records final answer to correct significant digits. Records the concentration with the appropriate units. The molar concentration I found was 0.1M.
Calculate the %(m/v) of hydrochloric acid using the molar concentration you found. Shows calculations. Records final answer to correct significant digits. Records the concentration with the appropriate units. The molar concentration I found was 0.1M.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter24: The Standardization Of A Basic Solution And The Determination Of The Molar Mass Of An Acid
Section: Chapter Questions
Problem 2ASA: In an acid-base titration, 21.16 mL of an NaOH solution are needed to neutralize 20.04 mL of a...
Related questions
Question
Calculate the %(m/v) of hydrochloric acid using the molar concentration you found.
Shows calculations. Records final answer to correct significant digits. Records the concentration with the appropriate units.
The molar concentration I found was 0.1M.

Transcribed Image Text:Calculate the average volume of NaOH used in your trials.
Correctly calculates the average amount of NaOH used.
Records the answer with proper units and significant digits.
Average of NAOH used:
(27.001+26.004+26.004)/3
= 26. 336
=30 mL
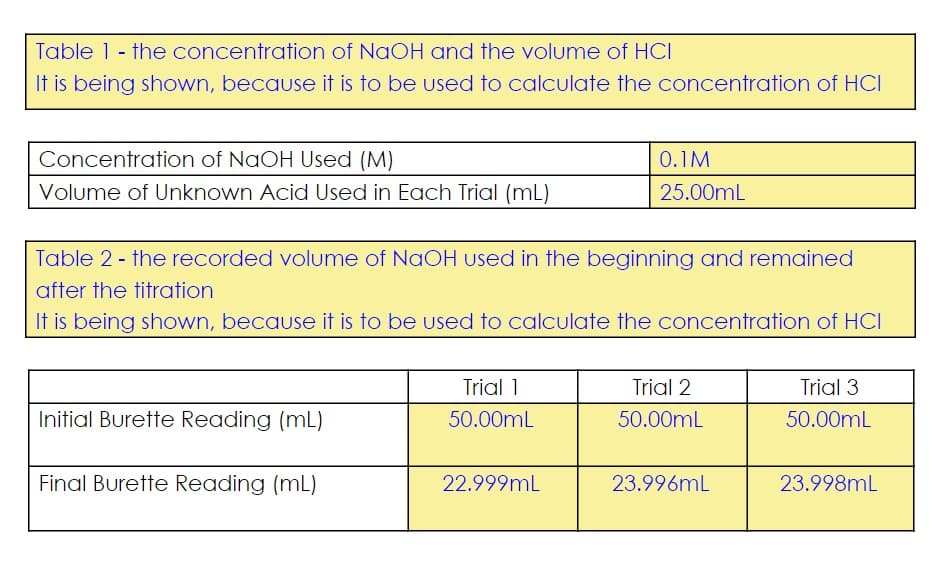
Transcribed Image Text:Table 1 - the concentration of NaOH and the volume of HCI
It is being shown, because it is to be used to calculate the concentration of HCI
Concentration of NaOH Used (M)
0.1M
Volume of Unknown Acid Used in Each Trial (mL)
25.00mL
Table 2 - the recorded volume of NaOH used in the beginning and remained
after the titration
It is being shown, because it is to be used to calculate the concentration of HCI
Trial 1
Trial 2
Trial 3
Initial Burette Reading (mL)
50.00mL
50.00mL
50.00mL
Final Burette Reading (mL)
22.999mL
23.996mL
23.998mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning