Calculate the pH at 25 °C of a 0.30M solution of potassium acetate (KCH,CO,). Note that acetic acid (HCH,CO,) is a weak acid with apK, of 4.76. Round your answer to 1 decimal place. pH =| ?
Calculate the pH at 25 °C of a 0.30M solution of potassium acetate (KCH,CO,). Note that acetic acid (HCH,CO,) is a weak acid with apK, of 4.76. Round your answer to 1 decimal place. pH =| ?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 2.1ACP: Phosphate ions are abundant in cells, both as the ions themselves and as important substituents on...
Related questions
Question
100%
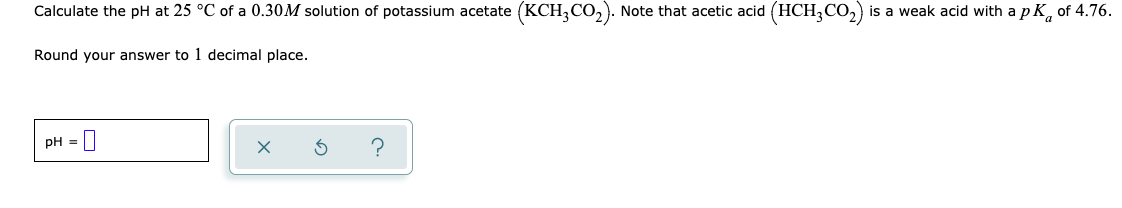
Transcribed Image Text:Calculate the pH at 25 °C of a 0.30M solution of potassium acetate (KCH,CO,). Note that acetic acid (HCH,CO,) is a weak acid with a p K, of 4.76.
Round your answer to 1 decimal place.
PH =||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning