Q: Reaction #2 Figure #2 Br compound A KOH, ethanol heat لمی میں نہیں ہیں compound B compound C…
A:
Q: Give the formula for the following ionic compounds. a. potassium sulfide b. aluminum phosphate c.…
A:
Q: The number of protons in the nucleus of an atom determines the species of the atom, i.e., the…
A:
Q: B. Draw the Lewis electron-dot structure for each ionic compound that will be formed by the…
A:
Q: 4. Calculate the equilibrium constant for the following systems: a. N₂(g) + 3 H₂(g) 2 NH3(g), where…
A: Equilibrium constant is ratio of concentration of products and reactants.
Q: 3. One component of the waste water that enters a waste water plant for treatment is urea, (NH2)2CO.…
A: The process involves the following steps:Urea hydrolysis: Urea reacts with water in the presence of…
Q: Use the References to access important values if needed for this question. 1 What is the total…
A: Lewis Structure is a representation of the valence shell electrons in a molecule. It is used to…
Q: Nitrogen monoxide and water react to form ammonia and oxygen, like this: 4 NO(g) + 6H₂O(g) - 4…
A: According to Le Chatelier's principle, if an equilibrium is disturbed by changing conditions, the…
Q: Use IUPAC rules to name the following molecule H₂C CH3 CH 3 CH3. is
A: IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as…
Q: For the cell shown, the measured cell potential, Ecell, is -0.3537 V at 25 °C. Pt(s) | H₂(g, 0.837…
A: Cell potential, Ecell= -0.3537 VHalf-cell reactions are also given.
Q: Draw the most stable chair conformation of β-D-galactopyranose. Name and draw the major product that…
A: We have to draw the most stable chair conformer of β-D-galactopyranose.
Q: Which name is correct for the following 3 dots molecule? OH CI a) 6-chloro-7-ethyl-3-decanol b)…
A: Rules for IUPAC naming:-Select the longest chain which contains carbon-carbon single bonds. Given…
Q: How much time does it take to reduce the concentration of NO2 by half by looking at the reaction…
A: Initial NO2 concentration = 0.05 MTime taken to reduce concentration to half = ?
Q: Rank this compounds in order of increasing solubility (1-lowest and 4-highest) 3-hexanone 1000…
A: 1.Solubility is define as maximum amount of substance dissolve in a given a solvent .2. Greater the…
Q: Predict the product, draw the mechanism, and plot the reaction coordinate diagram for the…
A: The reaction given in the question follows Free Radical Mechanism.This mechanism occurs only in the…
Q: H₂N-CH- answer: CH₂ OH 1 H____CH-C CH₂ OH 2 CHIC- CH₂ CH-CH CH3 3 -OH
A: A protein is formed through the peptide bond. These peptide bonds are formed by the amino acids. The…
Q: 2 Draw a Lewis structure for NH3. • Do not include overall ion charges or formal charges in your…
A: To draw the Lewis structure of a molecule we have to calculate the total number of valence electrons…
Q: 9) Which of the following does NOT describe a metal? A) good conductor of heat B) good conductor of…
A: a. Metals have tendency to loose electrons in order to attain stable nearest noble gas…
Q: Predict the product, draw the mechanism, and plot the reaction coordinate diagram for the S1…
A: A question based on reaction mechanism. An aliphatic substitution reaction is given whose mechanism…
Q: The vapor pressure of Benzen is its vapor pressure at 20.0 °C (AH 80.1 °C in 1 atm. Calculate for…
A:
Q: Calculate the pH of a 1.0 mol/L aqueous solution of sodium benzoate. Note: Only the benzoate ion…
A:
Q: What is the pH change when 40.0 mL of 0.100 M NaOH solution is added to 40 mL of 0.100 M H3PO4…
A: Volume of 0.1 M NaOH solution = 40 mLVolume of 0.1 M H3PO4 solution = 40 mL
Q: Carry out the following conversions. Report your answers to the correct number of significant…
A: One measurement can be converted from one unit to the other unit by conversion factor. It will be…
Q: What is the splitting pattern expected for the proton at C-2 in the following compound? HH He H H Br…
A: 1H NMR spectroscopy is used to determine the structure of unknown compound in organic chemistry. The…
Q: Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are…
A: Precipitation reaction:In precipitation reaction and insoluble salt is formed by the reaction…
Q: How many isoprene units are there in mycophenolic acid? and What is it called? (i want clear labeled…
A: A naturally occurring substance called mycophenolic acid (MPA) is generated from the fungus…
Q: Each of 10 stereoisomeric sugar derivatives can be prepared via a multiple-step synthesis starting…
A: As 1L is the enantiomer of 1D, hence 1L is the non-superimposable mirror image of 1D.
Q: 3. C,H,O, is infinitely miscible (soluble) in water. Ethylene glycol is a nonelectrolyte that is…
A: The depression in the freezing point () is given by the formula, i is the van't hoff factorm is…
Q: Which do you predict has the higher boiling point and why? 1. Cl₂ II. Ar Because (Select all that…
A: The correct answer is:5) I, III, IV, V
Q: Identify the reducing agent in the reaction: Ag(s) + NO3(aq) → Ag*(aq) + NO(g) Ag¹(aq) Ag (s) O…
A: It is based on the concept of Redox reaction Here we are required to find the reducing agent in the…
Q: 10.0 g of a metal chloride salt is dissolved in 90.0 g H2O. This solution has a vapor pressure that…
A:
Q: Which of the following compounds is most acidic? 11 Br E III 2 IV OH
A: The stability of the conjugate base that is generated after the acidic chemical loses a proton in…
Q: Answer the following questions for the reaction scheme below: phenol pka-10.0 OH CEN cyanida a. The…
A: The equilibrium reaction given is
Q: Draw structural formulas for organic products A and B in the window below. CH3 CH3CHCH₂CH₂1 Li…
A:
Q: Predict the products from the following chemical reactions. HNO3 + Ba(OH)2 CaCl2 + CsOH…
A: 1) HNO3 + Ba(OH)2:The reaction between nitric acid (HNO3) and barium hydroxide (Ba(OH)2) is a double…
Q: 15.0 mL of 0.60 mol/L ascorbic acid (C6H8O6(aq)) is titrated with 0.60 mol/L KOH. Determine the pH…
A: Answer:-This question is answered by using the simple concept of calculation pH at the equivalence…
Q: Need must HANDWRITING THANKS. Dont copy paste. 2. Explain why we need to prepare a series of…
A: A question based on tools in analytical chemistry. Description about the standardization curve and…
Q: The first step in the reaction of Alka-Seltzer with stomach acid consists of one mole of sodium…
A:
Q: a. Name the following molecules: Br CI b. On the structures above, put an asterisk (*) on any…
A: a). 1. 5-isopropyl-3-methyloctane Chemical Formula: C12H26 Exact Mass: 170.20 Molecular Weight:…
Q: Question 10 Structure A Br ...C H3C\ H CH₂CH3 Structure B CH 3 Bric H CH₂CH3 Determine the…
A: The compounds with the same molecular formula but different bond connectivity are structural…
Q: Calculate the solubility of CuCO3 in water at 25 °C. You'll find Kp data in the ALEKS Data tab.…
A: From standard data, Ksp for CuCO3 = 1.4 × 10-10
Q: The reaction conditions to carry out the production of cyclopentene using bromocyclopentane as the…
A:
Q: what can you do to make an organic acid more soluble in water? For example propionic acid?
A: By adding a base such as sodium hydroxide or sodium bicarbonate to the mixture, the propionic acid…
Q: 4. The following Claisen condensation involves two different reactants. It is called a crossed…
A: The question is based on organic reactions. we need to identify the product formed and explain…
Q: Dont copy paste solve correct. . 3. Give at least three factors that you must consider when choosing…
A: Instrument for analysis is used to determine, characterize and quantify chemical components in…
Q: The solubility of O₂ in water at 25°C is 3.16 g/mL when the gas pressure is maintained at 1.00 atm.…
A:
Q: d) In the following oxidation-reduction reaction, Ecell is measured as 0.12 V in the electrochemical…
A:
Q: Can you explain 4b? The answer is iodine is oxidized and chlorine is reduced. I'm just not sure…
A: Oxidation reaction proceeds by increase the oxidation number. Reduction reaction proceeds by…
Q: If the original Ca(OH) 2 solution used in the K sp lab was not saturated in Part 1 (not enough solid…
A: The question is based on the concept of solubility product principle. It that states that when a…
Q: Can you help me answer this?
A: The question is based on the concept of molarity. molarity is a method of expressing concentration…
Kindly show all your work for the following question:
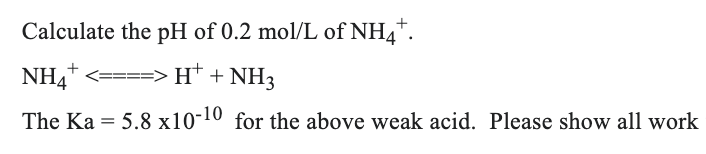
Step by step
Solved in 3 steps with 4 images

- The pH for 0.050 M CH3NH2 (Kb for CH3NH2 = 4.0 X 10^-4 )?What is the pH of the solution after mixing 0.171 g of Mg(OH)2 (MW=58.321 g/mol) with 68.9 mL of 0.0569 M HCl? The resulting solution was diluted to 100 mL. Round your calculated value for pH to two figures to the right of the decimal point.You start with a 10.0 mM solution of H3PO4 at a pH of 7.0. If you were to add 2.0 mL of 100 mM HCl what would the resulting pH be? Please show work.
- Kb for methylamine at 25°C is 4.4 x 10-4 what is its pH if 0.10M solution is used.When calculating the PH for a .023 mole solution of HBrO2. My Ki is 2.1x10-4 and PH is 2.6. However Calculate the PH for a solution that is .023 M in HBrO2 and .03M in Ca(BrO2)2? Ki for HBrO2 = 2.1x10-4 Will that increase my PH?Hello, can someone please help me with these chem questions and give me a clear step-by-step solution. Thank you very much for your help. a)The [H3O+], in M, of 10.00 mL of 0.100 M NaOH after adding 10.00 mL of indicator solution. To enter exponential values, use the format 1.0e-5. b)The [H3O+], in M, of 10.00 mL of 0.100 M HCl after adding 10.00 mL of the indicator solution. To enter exponential values, use the format 1.0e-5.
- Calculate the pH of a weak acid which dissociates as AH ⇌ A- + H+ ( like CH3COOH ⇌ CH3COO- + H+ or HNO2 ⇌ NO2- + H+ ) knowing that the initial concentrations are [AH]0 0.11 M and the acid dissociation constant, Ka , is 5.86e-7 pH _______ ← please insert your valueThe value for Kw at 0.0°C is 1.14 x 10^-15. Calculate the pH for a neutral solution at this temperature.What is the pH of a solution that has a [OH-] of 8.5 x 10-4 please show work
- v. What is pH? Calculate the pH for 0.0001N NH4OH solution of 100mL volume. The pKb value for NH4OH at 25oC is 1.76 x 10-5.calculate the PH of 1.5 M solution of hydroxylamine at 25 degrees C with a Kb = 9.1 times 10^-9Can you please answer A-C and show all of the steps to the solution please and thank you

