EXERCISES A. What mass of copper can be made by reducing 16g of copper oxide with hydrogen? Equation Cuo + H2 = Cu + H2O 16g 80 Masses Mr 64 Mass of Cu B. What mass of Magnesium will react with 19.6 g of sulphuric acid? Equation Mg + H2SO4 = MgSO4 + H2 Masses 19.6g
EXERCISES A. What mass of copper can be made by reducing 16g of copper oxide with hydrogen? Equation Cuo + H2 = Cu + H2O 16g 80 Masses Mr 64 Mass of Cu B. What mass of Magnesium will react with 19.6 g of sulphuric acid? Equation Mg + H2SO4 = MgSO4 + H2 Masses 19.6g
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section9.3: Mass Calculations
Problem 9.5SC: ercise 9.5 In Example 9.6 we answered one of the questions we posed in the introduction to this...
Related questions
Question
Can you please answer A-C and show all of the steps to the solution please and thank you
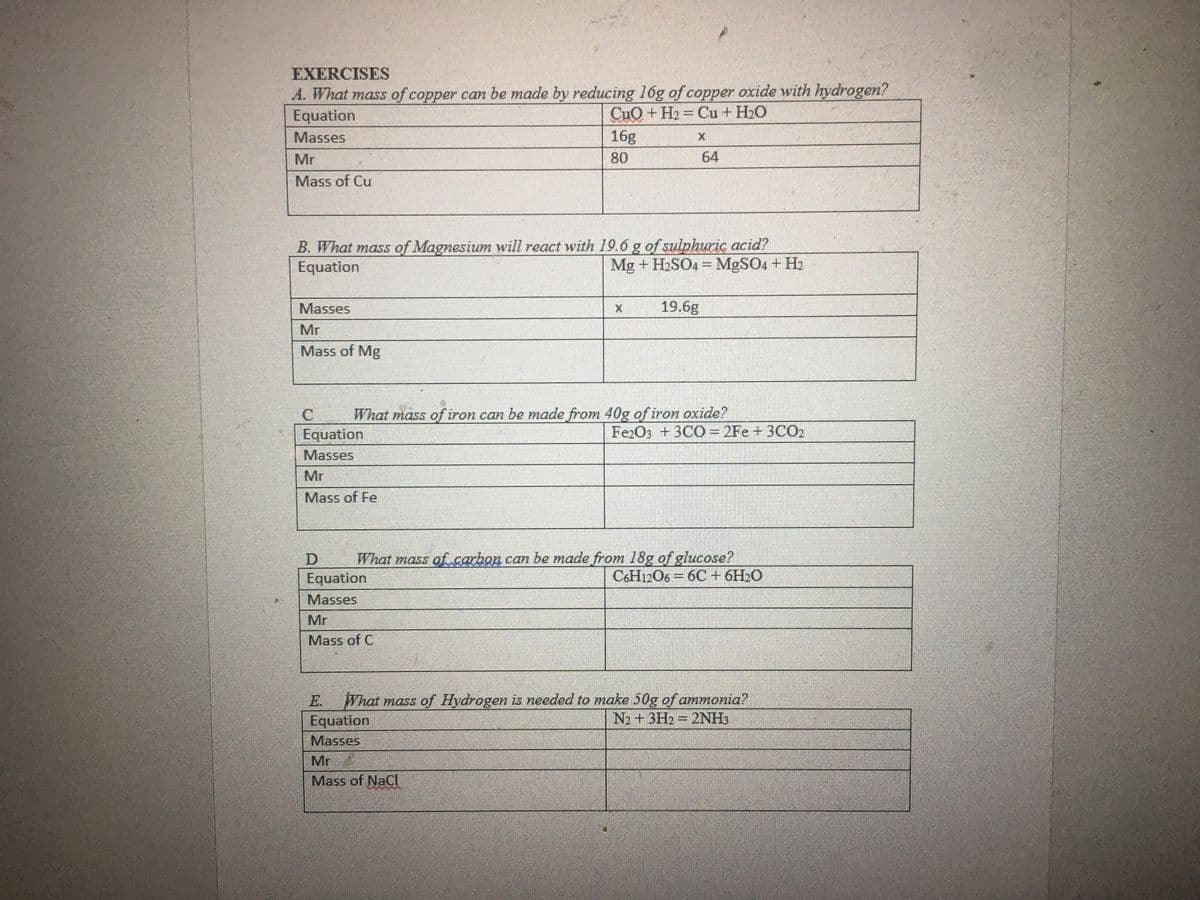
Transcribed Image Text:EXERCISES
A. What mass of copper can be made by reducing 16g of copper oxide with hydrogen?
Equation
CuO + H2 = Cu + H2O
Masses
16g
Mr
80
64
Mass of Cu
B. What mass of Magnesium will react with 19.6 g of sulphuric acid?
Equation
Mg + H2SO4 = MgSO4+ H2
Masses
19.6g
Mr
Mass of Mg
What mass of iron can be made from 40g of iron oxide?
Fe2O3 +3CO= 2Fe + 3CO2
Equation
Masses
Mr
Mass of Fe
What mass ofcarbon can be made from 18g of glucose?
C&H12O6 = 6C+ 6H O
Equation
Masses
Mr
Mass of C
E. What mass of Hydrogen is needed to make 50g of ammonia?
Equation
Masses
N2+3H2 2NH3
Mr
Mass of NaCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning