Calculate the pH of 1.00 L of the buffer 1.08 M CH3COONA/0.97 M CH3COOH before and after the addition of the following species. (Assume there is no change in volume.) (a) pH of starting buffer: (b) pH after addition of 0.080 mol NaOH: (c) pH after further addition of 0.121 mol HCl:
Calculate the pH of 1.00 L of the buffer 1.08 M CH3COONA/0.97 M CH3COOH before and after the addition of the following species. (Assume there is no change in volume.) (a) pH of starting buffer: (b) pH after addition of 0.080 mol NaOH: (c) pH after further addition of 0.121 mol HCl:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 71QAP: The species called glacial acetic acid is 98% acetic acid by mass (d=1.0542g/mL). What volume of...
Related questions
Question
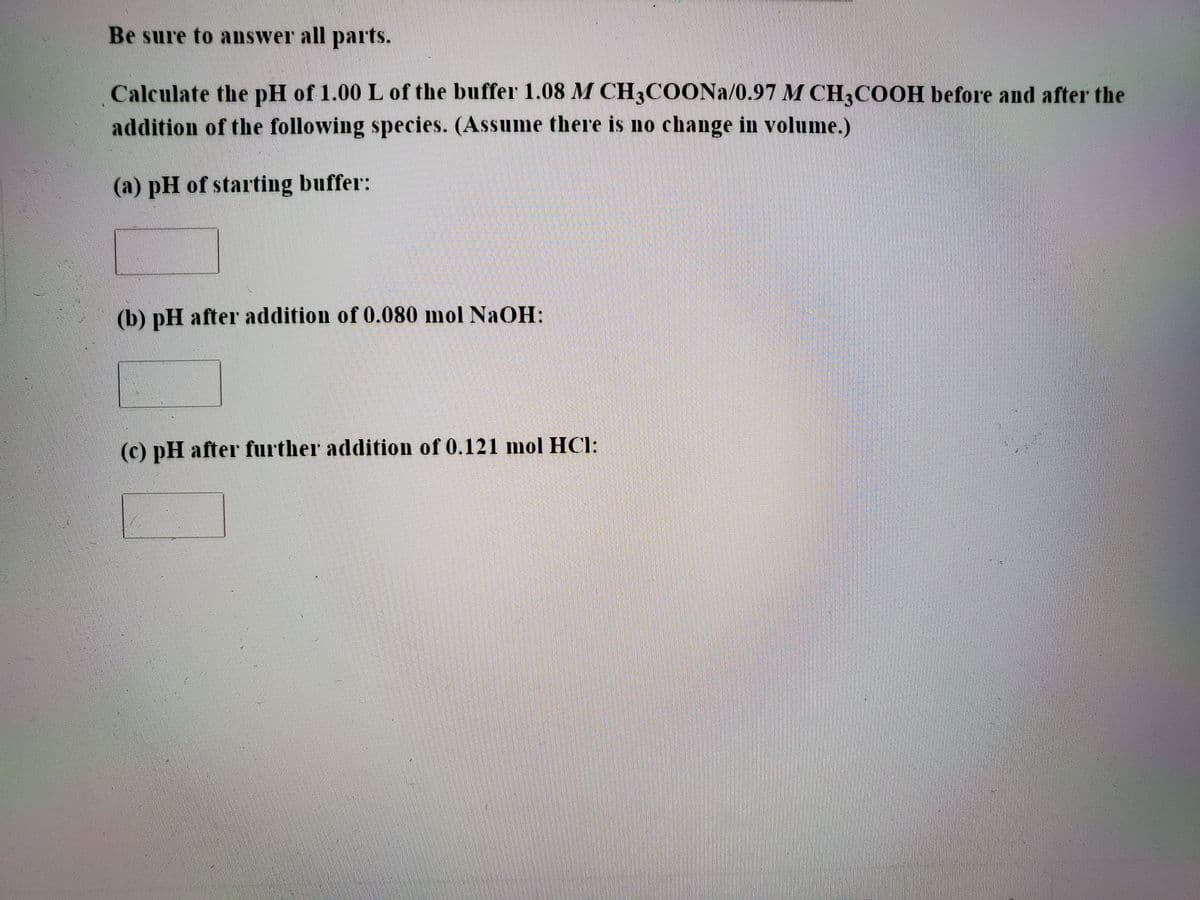
Transcribed Image Text:Be sure to answer all parts.
Calculate the pH of 1.00 L of the buffer 1.08 M CH3COONA/0.97 M CH3COOH before and after the
addition of the following species. (Assume there is no change in volume.)
(a) pH of starting buffer:
(b) pH after addition of 0.080 mol NaOH:
(c) pH after further addition of 0.121 mol HCl:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning