An alternative way of preparing the imidazolium buffer involves preparing solutions of imidazole (A) and imidazolium (HA; supplied by the manufacturer as a hydrobromide salt with a molar mass of 149g mol) and then combining the solutions ta.obtain a buffer with the desired pH and concentration. Calculate the mass (g) of imidazole (A) and imidazolium (HA) needed to prepare 100ml of the 1.0M stock solutions. Include 1 digit to the right of the decimal. mass HA (g) mass A (g) =
An alternative way of preparing the imidazolium buffer involves preparing solutions of imidazole (A) and imidazolium (HA; supplied by the manufacturer as a hydrobromide salt with a molar mass of 149g mol) and then combining the solutions ta.obtain a buffer with the desired pH and concentration. Calculate the mass (g) of imidazole (A) and imidazolium (HA) needed to prepare 100ml of the 1.0M stock solutions. Include 1 digit to the right of the decimal. mass HA (g) mass A (g) =
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 82CP
Related questions
Question
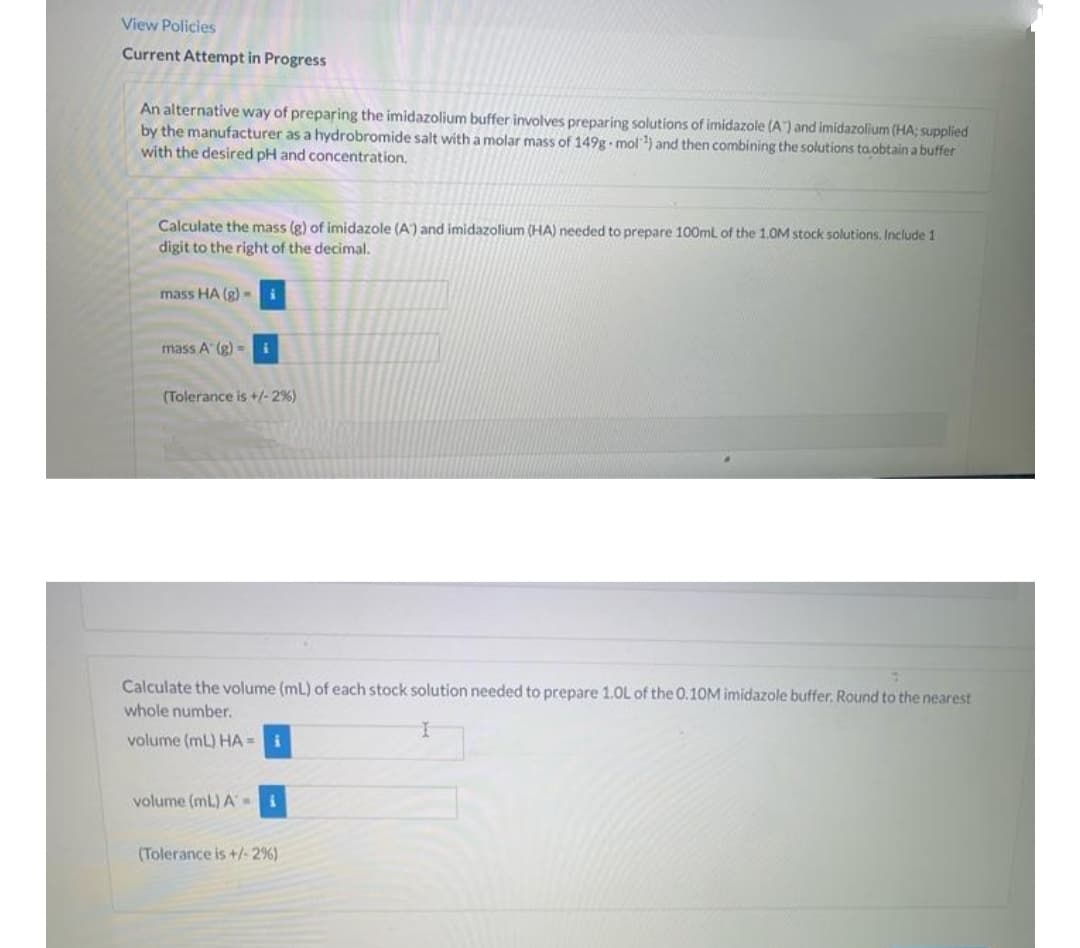
Transcribed Image Text:View Policies
Current Attempt in Progress
An alternative way of preparing the imidazolium buffer involves preparing solutions of imidazole (A) and imidazolium (HA; supplied
by the manufacturer as a hydrobromide salt with a molar mass of 149g mol) and then combining the solutions ta.obtain a buffer
with the desired pH and concentration.
Calculate the mass (g) of imidazole (A) and imidazolium (HA) needed to prepare 100ml of the 1.0M stock solutions. Include 1
digit to the right of the decimal.
mass HA (g) -i
mass A (g) =i
(Tolerance is +/-2%)
Calculate the volume (ml) of each stock solution needed to prepare 1.0L of the 0.10M imidazole buffer. Round to the nearest
whole number.
volume (ml) HA = i
volume (mL) A=
(Tolerance is +/- 2%)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
