Calculate the pH of a blood plasma sample with a total CO₂ concentration of 25.7 mM and bicarbonate concentration of 24.4 mM. The relevant pK, of carbonic acid is 6.1. Enter the answer with three significant figures.
Calculate the pH of a blood plasma sample with a total CO₂ concentration of 25.7 mM and bicarbonate concentration of 24.4 mM. The relevant pK, of carbonic acid is 6.1. Enter the answer with three significant figures.
Chapter13: Dimensional Analysis/units Conversion
Section: Chapter Questions
Problem 2.4P
Related questions
Question
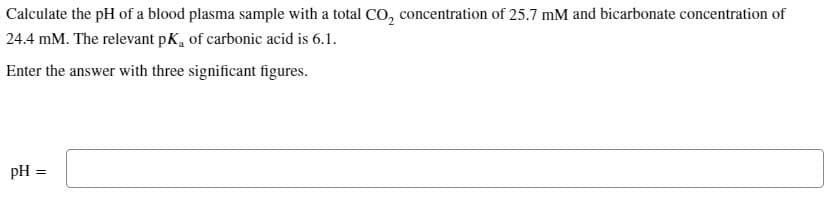
Transcribed Image Text:Calculate the pH of a blood plasma sample with a total CO₂ concentration of 25.7 mM and bicarbonate concentration of
24.4 mM. The relevant pK, of carbonic acid is 6.1.
Enter the answer with three significant figures.
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning



Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning


