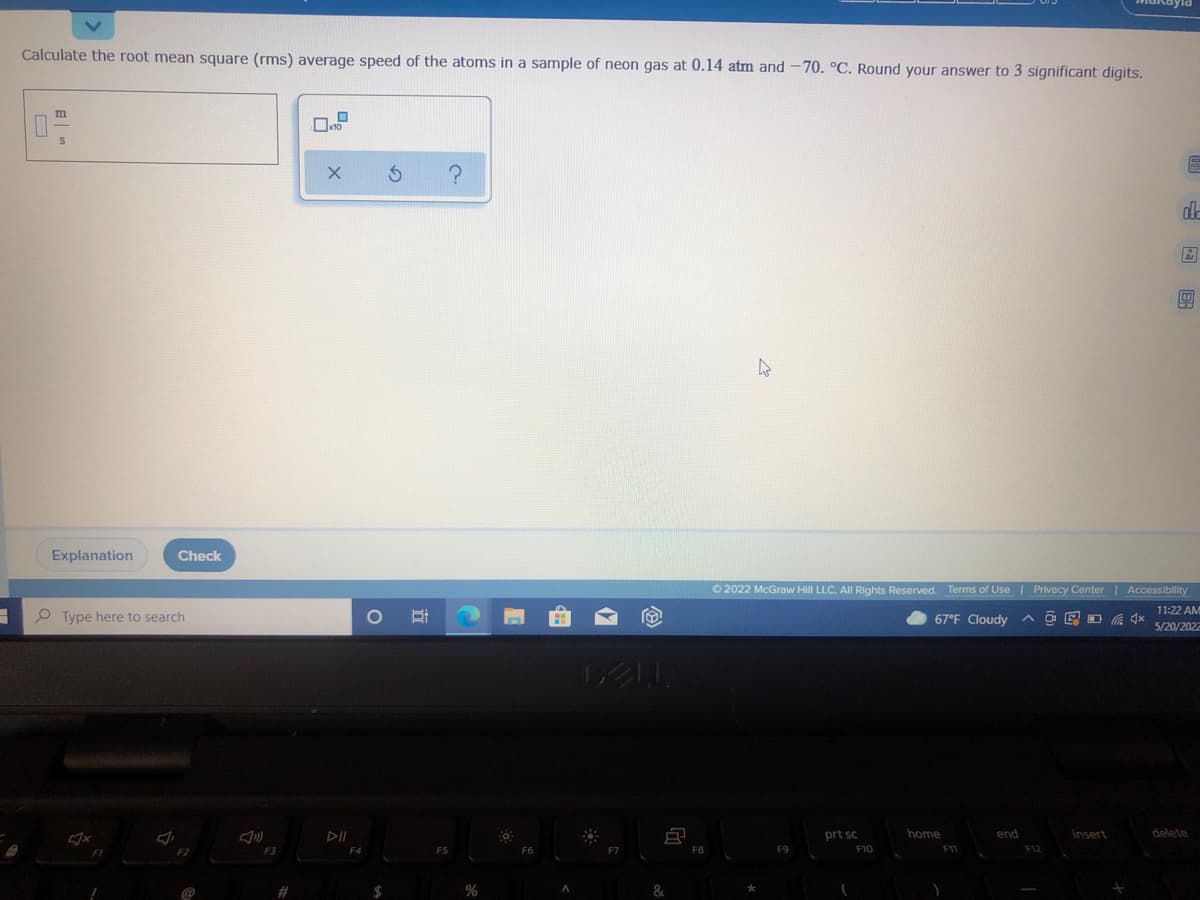
Calculate the root mean square (rms) average speed of the atoms in a sample of neon gas at 0.14 atm and -70. °C. Round your answer to 3 significant digits. m 0- x10 E X S ?
Calculate the root mean square (rms) average speed of the atoms in a sample of neon gas at 0.14 atm and -70. °C. Round your answer to 3 significant digits. m 0- x10 E X S ?
Chapter5: Gases
Section: Chapter Questions
Problem 74E: Urea (H2NCONH2) is used extensively as a nitrogen source in fertilizers. It is produced commercially...
Related questions
Question
Calculate the root mean square average speed of the Adams in a sample of neon gas at 0.14 atm and -70 degrees Celsius. Round your answer to 3 significant digits
Transcribed Image Text:H
Calculate the root mean square (rms) average speed of the atoms in a sample of neon gas at 0.14 atm and -70. °C. Round your answer to 3 significant digits.
m
0-
x10
E
400
X Ś ?
ol
Ar
00
h
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
O Ai
67°F Cloudy@
D4x
11:22 AM
5/20/2022
end
delete
S
Explanation
Type here to search
F1
F2
Check
F3
F4
F5
%
F6
A
DELL
F7
8
&
F8
*
F9
prt sc
(
F10
home
F11
F12
insert
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax