Calculate the standard enthalpy of formation of C10H10. Its standard enthalpy of combustion is shown by the equation: 2C10H10 (l) + 25O2 (g) → 20CO2 (g) + 10H2O (l) ∆H = 10,314 KJ/mol The standard heat of formation (KJ/mol) at 298.15oC : CO2 = -393.5 ; H2O(l) = -285.8 2. Determine ΔG° at 298.15K for the reaction: 4Fe (s) + 3O2 (g) → 2Fe2O3 (s) Given that ΔH° = -1648 kJ ; ΔS° = -549.3 J/K 3.
Calculate the standard enthalpy of formation of C10H10. Its standard enthalpy of combustion is shown by the equation: 2C10H10 (l) + 25O2 (g) → 20CO2 (g) + 10H2O (l) ∆H = 10,314 KJ/mol The standard heat of formation (KJ/mol) at 298.15oC : CO2 = -393.5 ; H2O(l) = -285.8 2. Determine ΔG° at 298.15K for the reaction: 4Fe (s) + 3O2 (g) → 2Fe2O3 (s) Given that ΔH° = -1648 kJ ; ΔS° = -549.3 J/K 3.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
1. Calculate the standard enthalpy of formation of C10H10. Its standard enthalpy of combustion is shown by the equation: 2C10H10 (l) + 25O2 (g) → 20CO2 (g) + 10H2O (l)
∆H = 10,314 KJ/mol
The standard heat of formation (KJ/mol) at 298.15oC : CO2 = -393.5 ; H2O(l) = -285.8
2. Determine ΔG° at 298.15K for the reaction: 4Fe (s) + 3O2 (g) → 2Fe2O3 (s) Given that ΔH° = -1648 kJ ; ΔS° = -549.3 J/K
3. In the picture, Find the the ∆H°rxn if ∆G°rxn is 420 kJ/mol and ∆S°rxn is -143 J/mol*k
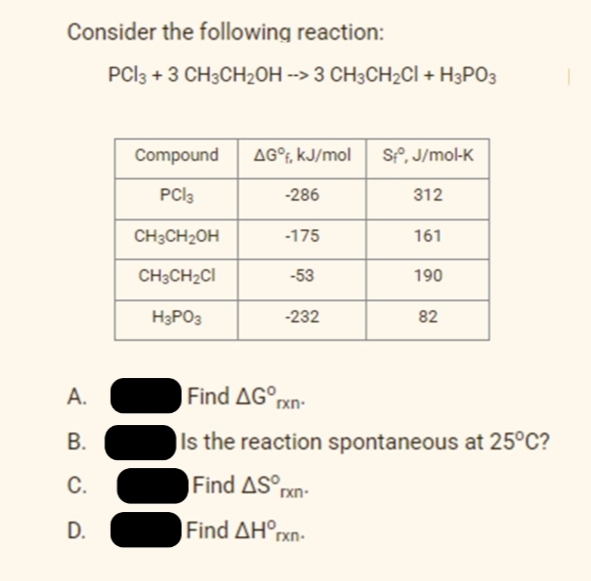
Transcribed Image Text:Consider the following reaction:
PCI3 + 3 CH3CH2OH --> 3 CH3CH2CI + H3PO3
Compound
AG°F, kJ/mol
SP, J/mol-K
PCI3
-286
312
CH3CH2OH
-175
161
CH3CH2CI
-53
190
H3PO3
-232
82
A.
Find AG rxn-
В.
ls the reaction spontaneous at 25°C?
C.
Find AS°pxn-
D.
Find ΔΗn
rxn-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

