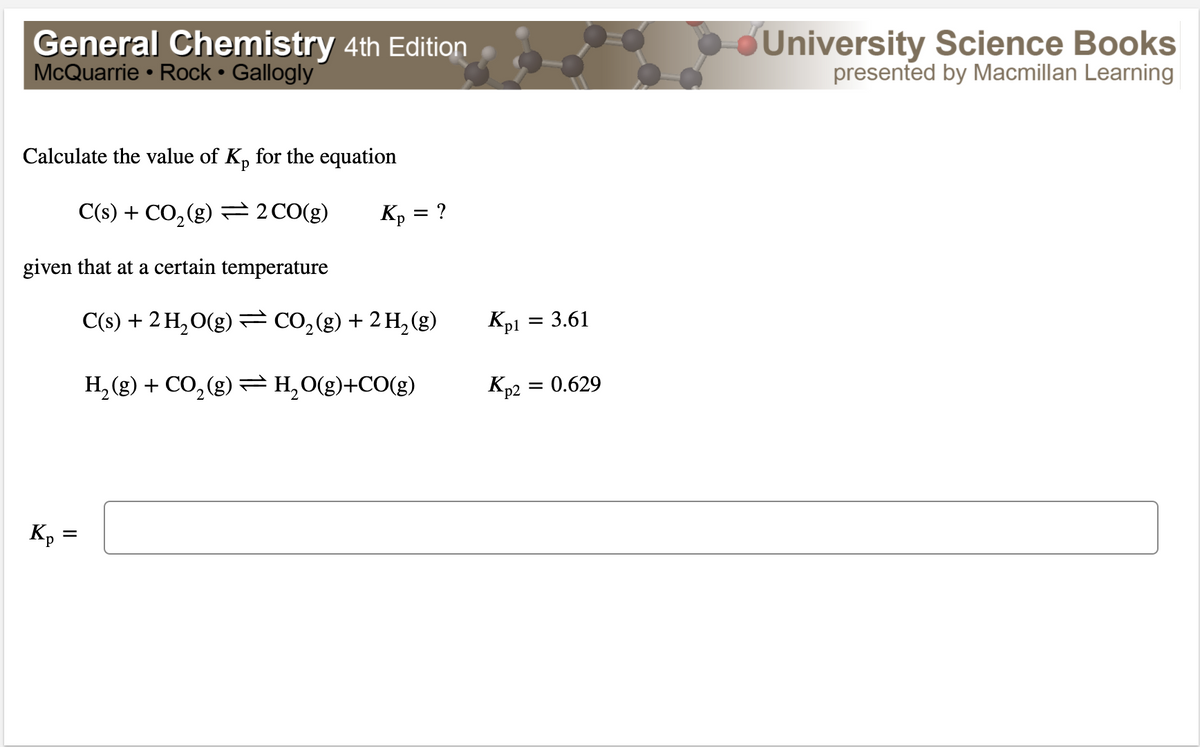
Calculate the value of K, for the equation C(s) + CO, (g) = 2CO(g) Kp = ? given that at a certain temperature C(s) + 2 H,O(g) = CO,(g) + 2 H, (g) Kp1 = 3.61 H, (g) + CO,(g) = H,O(g)+CO(g) Kp2 = 0.629
Calculate the value of K, for the equation C(s) + CO, (g) = 2CO(g) Kp = ? given that at a certain temperature C(s) + 2 H,O(g) = CO,(g) + 2 H, (g) Kp1 = 3.61 H, (g) + CO,(g) = H,O(g)+CO(g) Kp2 = 0.629
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section16.6: Gibbs Free Energy
Problem 16.10CE
Related questions
Question
Transcribed Image Text:General Chemistry 4th Edition
McQuarrie • Rock Gallogly
University Science Books
presented by Macmillan Learning
Calculate the value of K, for the equation
d.
C(s) + CO, (g) =2CO(g)
:?
KP
given that at a certain temperature
C(s) + 2 H,O(g) = CO,(g) + 2 H, (g)
Kpi = 3.61
Кр
0.629
H, (g) + CO, (g) =H,0(g)+CO(g)
Kp =
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning