Calculate the volume in milliliters of a 2.0 mol/L nickel(II) chloride solution that contains 200. g of nickel(II) chloride (NIC1,). correct number of significant digits. Be sure your answer has the O mL
Calculate the volume in milliliters of a 2.0 mol/L nickel(II) chloride solution that contains 200. g of nickel(II) chloride (NIC1,). correct number of significant digits. Be sure your answer has the O mL
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 82QAP: If a solid block of glass, with a volume of exactly 100 in.3, is placed in a basin of water that is...
Related questions
Question
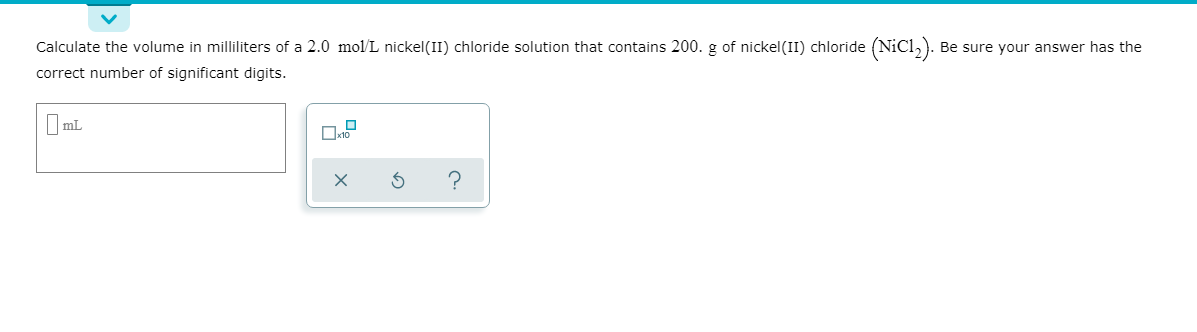
Transcribed Image Text:Calculate the volume in milliliters of a 2.0 mol/L nickel(II) chloride solution that contains 200. g of nickel(II) chloride (NIC1,).
correct number of significant digits.
Be sure your answer has the
O mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning