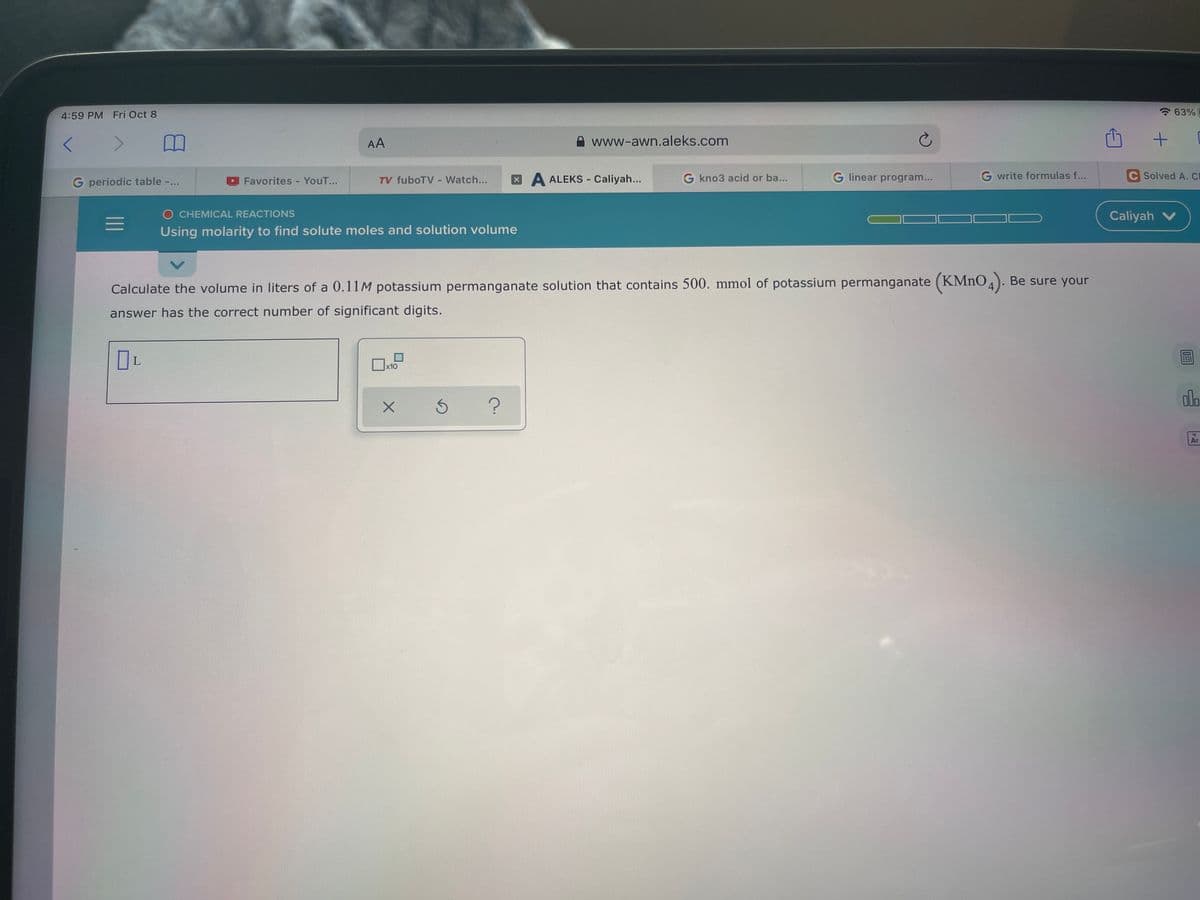
Calculate the volume in liters of a 0.11M potassium permanganate solution that contains 500. mmol of potassium permanganate (KMNO4). Be sure your answer has the correct number of significant digits. da
Calculate the volume in liters of a 0.11M potassium permanganate solution that contains 500. mmol of potassium permanganate (KMNO4). Be sure your answer has the correct number of significant digits. da
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 9P
Related questions
Question
Transcribed Image Text:4:59 PM Fri Oct 8
63%
AA
A www-awn.aleks.com
G periodic table -..
Favorites - YouT...
TV fuboTV - Watch...
XA ALEKS - Caliyah...
G kno3 acid or ba...
G linear program...
G write formulas f...
C Solved A. CI
O CHEMICAL REACTIONS
Caliyah V
Using molarity to find solute moles and solution volume
Calculate the volume in liters of a 0.11M potassium permanganate solution that contains 500. mmol of potassium permanganate (KMNO4). Be sure your
answer has the correct number of significant digits.
x10
alo
18
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning