calculated from of formation of the substances involved in the reaction: AHxn=AH (products) - AH (reactants) Entropy change, AS, is a measure of the number of energetically equivalent microstates introduced into the system during the reaction. The degree of spontaneity of a reaction is represented by the Gibbs free energy, AG. The Gibbs free energy depends on both the enthalpy and entropy changes. ▼ Hint 1. How to approach the problem In the previous part, you determined that the enthalpy AHxn was 229 kJ for the reaction written. This means that 229 kJ of heat is absorbed when exactly 2 mol of A and 1 mol of react to produce 2 mol of C and 2 mol of D according to the balanced chemical equation 2A + B 2C+ 2D When less A reacts, less heat is absorbed. When more A reacts, more heat is absorbed. S up the heat absorbed to correspond to 2.60 mol of A.
calculated from of formation of the substances involved in the reaction: AHxn=AH (products) - AH (reactants) Entropy change, AS, is a measure of the number of energetically equivalent microstates introduced into the system during the reaction. The degree of spontaneity of a reaction is represented by the Gibbs free energy, AG. The Gibbs free energy depends on both the enthalpy and entropy changes. ▼ Hint 1. How to approach the problem In the previous part, you determined that the enthalpy AHxn was 229 kJ for the reaction written. This means that 229 kJ of heat is absorbed when exactly 2 mol of A and 1 mol of react to produce 2 mol of C and 2 mol of D according to the balanced chemical equation 2A + B 2C+ 2D When less A reacts, less heat is absorbed. When more A reacts, more heat is absorbed. S up the heat absorbed to correspond to 2.60 mol of A.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 84AP
Related questions
Question
Part b For the reaction given in Part A, how much heat is absorbed when 2.60 mol of A reacts?
And part c said At what temperature Teq do the forward and reverse corrosion reactions occur in equilibrium?

Transcribed Image Text:Chemical energy is released or absorbed from
reactions in various forms. The most easily
measurable form of energy comes in the form of
heat, or enthalpy. The enthalpy of a reaction can be
calculated from the heats of formation of the
substances involved in the reaction:
AHxn = AH (products) - AH (reactants)
Entropy change, AS, is a measure of the number
of energetically equivalent microstates introduced
into the system during the reaction. The degree of
spontaneity of a reaction represented by the
Gibbs free energy, AGO. The Gibbs free energy
depends on both the enthalpy and entropy changes
that take place during the reaction:
ΔG° = ΔΗ° -ΤΔS
where T is standard temperature, 298 K.
Part B
For the reaction given in Part A, how much heat is absorbed when 2.60 mol of A reacts?
Express your answer numerically in kilojoules.
▾ View Available Hint(s)
▼
▼
Review | Constants | Periodic Table
Hint 1. How to approach the problem
In the previous part, you determined that the enthalpy AH was 229 kJ for the reaction as
written. This means that 229 kJ of heat is absorbed when exactly 2 mol of A and 1 mol of B
react to produce 2 mol of C and 2 mol of D according to the balanced chemical equation
2A + B 2C + 2D
When less A reacts, less heat is absorbed. When more A reacts, more heat is absorbed. Scale
up the heat absorbed to correspond to 2.60 mol of A.
Hint 2. Determine the heat absorbed per mole of A
Given the enthalpy of this reaction as written
2A + B 2C + 2D,
how much heat is absorbed for each mole of A that reacts?
AHxn = 229kJ
Transcribed Image Text:Chemical energy is released or absorbed from
reactions in various forms. The most easily
measurable form of energy comes in the form of
heat, or enthalpy. The enthalpy of a reaction can be
calculated from the heats of formation of the
substances involved in the reaction:
AHxn = AH; (products) - AH; (reactants)
Entropy change, AS, is a measure of the number
of energetically equivalent microstates introduced
into the system during the reaction. The degree of
spontaneity of a reaction is represented by the
Gibbs free energy, AGO. The Gibbs free energy
depends on both the enthalpy and entropy changes
that take place during the reaction:
AG=AH - TAS°
where I is standard temperature, 298 K.
▼
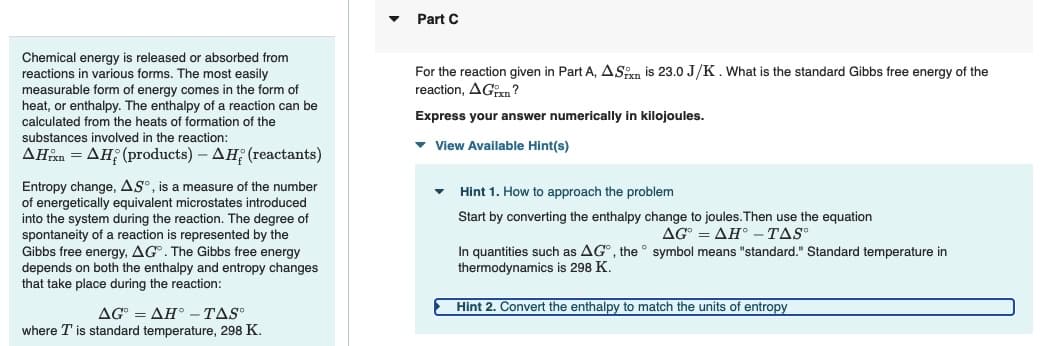
Part C
For the reaction given in Part A, ASn is 23.0 J/K. What is the standard Gibbs free energy of the
reaction, AG?
Express your answer numerically in kilojoules.
▾ View Available Hint(s)
Hint 1. How to approach the problem
Start by converting the enthalpy change to joules.Then use the equation
AG=AH° - TAS
symbol means "standard." Standard temperature in
In quantities such as AG, the
thermodynamics is 298 K.
Hint 2. Convert the enthalpy to match the units of entropy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning