Q: At 148.9 °C, the Keq for the reaction: 1 A + 3 B +> 5 AB is 4.19 · 10-5 What is K,? Enter your…
A:
Q: Barium sulphate is a relatively insoluble salt used for medical radiographs of the gastrointestinal…
A:
Q: Calculate AHo for the reaction: 20;Hlg) + 70x(g) - 4 CO:g) + 6 HO(g) If. CHi(g) + 30:(9) -2 CO:(g) +…
A:
Q: Do increasing CO2 concentrations follow temperatures or precede them?
A: Carbon dioxide is considered as one of the main greenhouse gases. It has a major impact on the…
Q: 13) What is Ksp? a) Ksp is a measure of how soluble a slightly soluble salt it. b) Ksp is…
A: Ksp (solubility product)
Q: Calculate the ΔGrxn for this reaction under these nonstandard conditions? 1 A (g) + 3 B (s) --> 2 C…
A: Given;we have to calculate the ∆Gr*n for this reactionnonstandard conditions1A(g)+3B(s)→2C(aq)…
Q: Temperature (°C) 25 C Write the Ksp expression here: Calculate the Ksp value here (show work):…
A:
Q: Which figure BEST represents the dissolution of MgCl2 in water? 0-0-H 2-H Ma H-O-H H H 0 HH MgCl₂ H…
A:
Q: Explain why there is no reaction for the situation (2) but there is a reaction in situation (1)?…
A:
Q: The concentration of S2- in a saturated solution of FeS is 6.17x10-10 mol/L at a certain…
A: We have to predict the Ksp of given salt.
Q: just to verify the answer rto 1 is increasing and 2 is increasing>??
A:
Q: Given the following chemical reaction in the gas phase: 2A(g) = B(g) + C(g) With: AHo = 27.0kJ y K =…
A: The given reaction is, 2A(g) <-----> B(g) + C(g) ΔH0 = 27.0 kJ K = [B] [C] /…
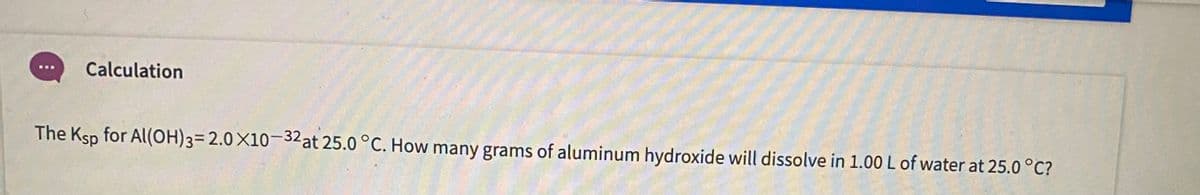
Q: 6.1 The solubility of aluminum hydroxide (Al(OH)3) is approximately 1.8 x 10-9 Mat a specific…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Approximately 1.5 × 10-3 g of iron(11) hydroxide, Fe(OH)2(s), dissolves per liter of water at 18°C.…
A: The mass of iron(II) hydroxide dissolved in 1 liter of water is = 1.5 x 10^-3 g The Ksp for water is…
Q: Temperature (°C) 25 C Write the Ksp expression here: Calculate the Ksp value here (show work):…
A:
Q: The disassociation of barium hydroxide in water is an exothermic process. Which of the following…
A: Concept: In an exothermic reaction, the energy of the product is lower than the energy of reactants.…
Q: The dissolution of NH4Cl in water is an endothermic process. If the initial temperature of water is…
A: Interpretation - To determine the expected temperature of the aqueous system when the dissolution…
Q: Calculate AG at 25°C for the following reaction: CO(g) + 2H2(g) → CH;OH(I) AG°xn = -2.9x10ʻ J/mol…
A: It is given that the initial pressure of CO is 5.0 atm and of H2 is 3.0 atm, and the reaction is-…
Q: In the following reaction calculate and find the normality when it is 1.0 M H3PO4? H3PO4 + 2NaOH →…
A: Given: Molarity of H3PO4 = 1.0 M
Q: 1. A student conducts an investigation to determine the heat of neutralization when 60.0 mL of 0.500…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: The equation for the dissolving of magnesium hydroxide is: Mg(O H)2(s) + heat Mg2+ (a q) + 20 H…
A: Each ionic compounds dissociates in their constituent ions when dissolved into a solvent. Hence…
Q: The solubility of calcium sulfate, CaSO4 in water is 0.0049 mol/L. Calculate Ksp.
A: Given : The solubility of CaSO4 in water is 0.0049 mol/L Hence 0.0049 mol/L concentration of CaSO4…
Q: Calculate the solubility of AgCl in water at 25 °C. You'll find K data in the ALEKS Data tab. sp…
A: Answer:- This question is answered by using the simple concept of calculation of solubility using…
Q: In our data set from our first run [0.050M] we found k' was 5.10 x 10^-3 s^-1; k' for our second run…
A: A question based on kinetics for the reactions in solution that is to be accomplished.
Q: (a) Write a chemical equation that describes the attack ofacid rain on limestone, CaCO3. (b) If a…
A: (a) Acid rain contains sulphuric acid that is formed when rain water dissolves sulfur dioxide,…
Q: Considering the Ksp value for the substance measured at 25 ° C, write the Ksp equation and…
A:
Q: The molar solubility of Mg(OH) 2 in water is 8.9x10-12 mol/L. What is the solubility product (Ksp)…
A: The dissociation equation for given compound is Mg(OH)2⇋Mg2++2OH-. The given molar solubility, s…
Q: 1. A student performed this same experiment and obtained the following data: Temperature of hot…
A: Temperature of hot water = 97.5oC =97.5+273.15=370.65 K Volume of water pulled into flask =125 mL…
Q: For the reaction 2H(g)H2(g), O AH = 0 and AS> 0. O AH> 0 and AS< 0 AHS and AS < 0
A: Chemical Reaction :: 2 H (g) ---> H2 (g) ∆H = ? ∆S = ?
Q: When a solution is supersaturated the concentration of the solute is _____ the solubility, and _____…
A: 1. Supersaturated solution means a solution where solute is present slightly more than its…
Q: Temperature C 25 °C Write the Ksp expression here: Calculate the Ksp value here (Show work): Volume…
A: The solubility product constant of a reaction is calculated by the multiplication of molarity of the…
Q: The slightly soluble salt Strontium iodate, Sr(IO3) 2 , dissolves in water as follows: Sr(IO3)2 =…
A:
Q: 2. _H,O, heat +_0+Ho B. Addition of water? C. Removal of dioxide? A. Removal of heat? D. Increase…
A: Balanced equation: 2H2O(l) + ∆ ⇋ O2(g) + 2H2(g) According to Le Chatlier's principle: If there is…
Q: (Q55) Nickel carbonate is being precipitated out of a 1.00 L solution. If the concentration of…
A:
Q: (Q55) Nickel carbonate is being precipitated out of a 1.00 L solution. If the concentration of…
A: Given that, Nickel carbonate , NiCO3 is precipitated out Volume of solution = 1.00 L Concentration…
Q: (Q50) What is the molar solubility of mercury (I) sulfate at 25°C? The Ksp of mercury (I) sulfate is…
A: Mercury (I) sulfate : Hg2SO4 ⇌2Hg+ + SO42- Let molar solubility is = S So, Ksp = [2Hg+]2[SO42-]…
Q: At 25°C, Keq = 4.8 x 10-6 for the balanced decomposition reaction of 2 moles of ICI. 2ICI(g) 1₂(g) +…
A:
Q: 6.) The dissolution of barium sulfate in water at 25.0 °C has a Ksp value of 1.1 X 10-10. If solid…
A: The chemical equation for the dissolution of BaSO4 in water is shown in equation (1).
Q: Temperature C 25 °C Write the Ksp expression here: Calculate the Ksp value here (Show work): Volume…
A: The solubility product constant is the multiplication of molarity of different constituting ions…
Q: Reaction: X →Y Reaction Coordinate Potential Energy (kJ/mol)
A: Heat of reaction is the difference in energy between the reactant and product.
Q: At 100°C, water dissolves 1.8 × 10-2 g of AgCl per liter. Compute the Ksp of AgCl at this…
A: Molar mass of AgCl = 143.2 gmol-1 Solubility in terms of moles/L can be calculated as: s = ((1.8 ×…
Q: For the reaction N2(g) 2N(g), 10 AH> 0 and AS 0 and AS > 0 O AH 0 O AH 0.
A: Given reaction: N2 (g) → 2N (g) We have to find the ∆H and ∆S values according to the given options.
Q: Derive the equation: pH + pOH = pKw
A: at 25°c possibility of pH + pOH = pKw is true.
Q: what is the ΔG when - K constant = 1.2x103 - temperature = 40 degrees - R = 8.31
A: Gibbs Free Energy Equation, ∆G = ∆G° + RT(lnK) ∆G = Gibbs Free Energy ∆G° = Standard Gibbs Free…
Q: Suppose 50.00 mL of a 1 x 10^–5 M solution of lead(II) nitrate is mixed with 50.00 mL of a 1 x 10^–6…
A: Solubility product: It is equilibrium constant that defines the solubility nature of solute in…
Q: Calculate the solubility of AgCl in water at 25 °C. You'll find K data in the ALEKS Data tab. sp…
A:
Q: At 25 °C the solubility of lead(II) bromide is 2.70 x 104 mol/L. Calculate the value of Ksp at this…
A: Given : Molar solubility of PbBr2 i.e Lead (II) Bromide = 2.70 X 10-2 M It means 2.70 X 10-2 M…
Q: Write the equation and Ksp expression for the dissolution of nickel(II) phosphate in water (const.…
A:
Q: Considering the Ksp values for the substances measured at 25 ° C, write the Ksp equation and…
A:
Q: Construct the expression for Kc for the following reaction. 4 HCI(aq) + O:(g) = 2 H:O(1) + 2 Cl:(g)…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- 9.96 Most first aid "cold packs" are based on the endothermic dissolution of ammonium nitrate in water: NH4NO3(s)NH4+(aq)+NO3(aq) H= 25.69 kJ A particular cold pack contains 50.0 g of NH4NO3 and 125.0 g of water. When the pack is squeezed, the NH4NO3dissolves in the water. If the pack and its contents are initially at 24.0°C, what is the lowest temperature that this bag could reach? (Assume that the ammonium nitrate solution has a specific heat of 4.25J g-l K-l, and that the heat capacity of the bag itself is small enough to be neglected.)The following is a thought experiment. Imagine that you put a little water in a test tube and add some NaF crystals. Immediately after you add NaF, you observe that the crystals begin dissolving. The quantity of solid NaF decreases, hut before long, it appears that no more NaF is dissolving. The solution is saturated. The equation for the dissolution of NaF in water is NaF(s) —* Na (aq) + F~(aq). As NaF dissolves, what do you think happens to the rate of dissolution? Describe w hat is occurring on the molecular level. Assume that the reverse reaction, Na+(aq) + F“(aq) —* NaF(s), also occurs as the crystal dissolves. In other words, both dissolution and precipitation are taking place. When it appears that there is no more change in the quantin’ of NaF dissolving (the solution is saturated), w hat has happened to the rates of the forward and reverse reactions? Explain your answer.Approximately 0.17 g iron(II) hydroxide, Fe(OH)2(s), dissolves per liter of water at 20°C. Calculate Ksp for Fe(OH)2(s) at this temperature.
- The solubility of lead(II) chloride, PbCl2 (MM: 278.1 g/mol), in water at 60 °C is 1.94 g/L. a) Calculate the Ksp for lead(II) chloride.The solubility of magnesium oxalate, MgC2O4, in water is 0.0093 mol/L, Calculate Ksp.The solubility product, Ksp, for Aluminum hydroxide, Al(OH)3, is 1.9e-33 at 25°C. What is the molar solubility of Aluminum hydroxide in a solution containing 0.071M KOH at 25°C?
- . The solubility product of magnesium carbonate, MgCO3, has the value Ksp = 6. 82 × 10−6at 25°C. How many grams of MgCO3will dissolve in 1.00L of water?In the dissolving of table salt in water, Na+Cl-(s) ---> Na+(aq) + Cl-(aq), at 25 oC . deltaH _____ 0, because ______________ deltaS ______ 0, because _____________ deltaG ______ 0 , because _____________What is the correct Ksp expression for Lead(II) Chloride dissolving in water?

