Temperature (°C) 25 C Write the Ksp expression here: Calculate the Ksp value here (show work): Solid: Sr(103)2 Volume of distilled water Mass of solid (mL) (g) 100mL 150g Molarity of Sr²+ 4.2x10^-3 Molarity of 103* 8.4x10^3
Temperature (°C) 25 C Write the Ksp expression here: Calculate the Ksp value here (show work): Solid: Sr(103)2 Volume of distilled water Mass of solid (mL) (g) 100mL 150g Molarity of Sr²+ 4.2x10^-3 Molarity of 103* 8.4x10^3
Chapter27: Molecular Fluorescence Spectroscopy
Section: Chapter Questions
Problem 27.11QAP
Related questions
Question
100%
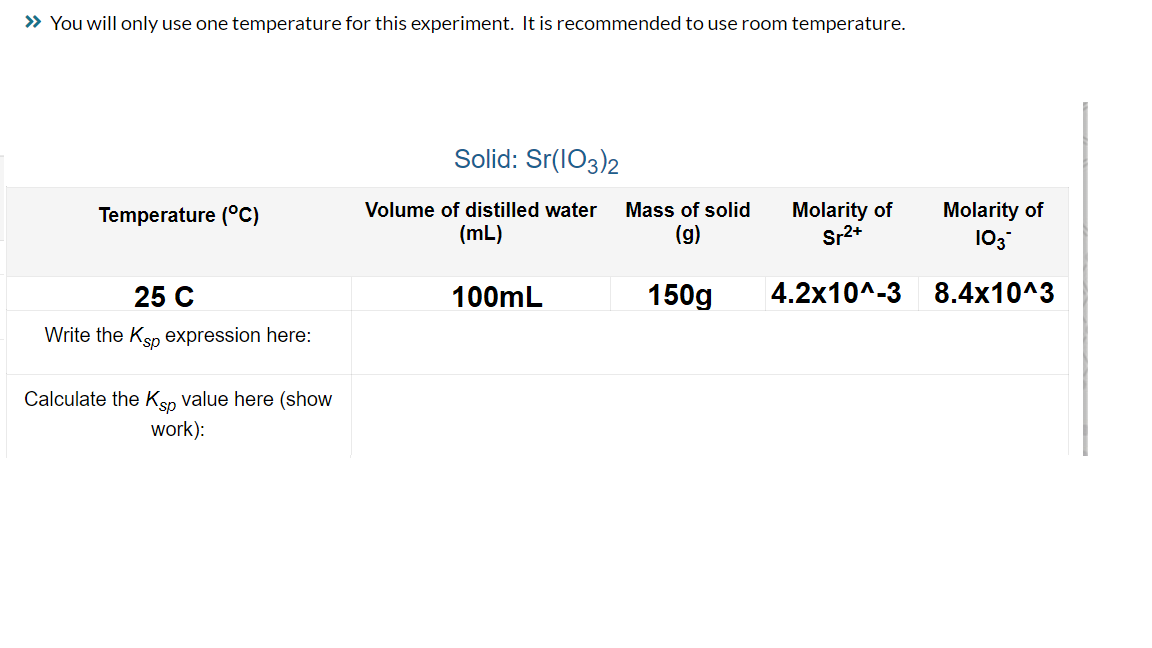
Transcribed Image Text:» You will only use one temperature for this experiment. It is recommended to use room temperature.
Temperature (°C)
25 C
Write the Ksp expression here:
Calculate the Ksp value here (show
work):
Solid: Sr(103)2
Volume of distilled water Mass of solid
(mL)
(g)
100mL
150g
Molarity of
Sr²+
4.2x10^-3
Molarity of
103*
8.4x10^3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



