Calorimeter Calibration 50 45 y = -0.44x + 45.86 40 A. 35 30 y = -0.35x + 34.533 Temperature 25 °C 20 y = -0.04x + 23.41 15 10 2 4 6. 8 Time (mins) Red=Temp as 5o mL of warm water slowly cooled over tim Green = Temp ar a mixture of both wam ahd cool water slouly cooled over me Rlue =Temp as 5o mL of cool water stayed relatively Ohstant over time
Calorimeter Calibration 50 45 y = -0.44x + 45.86 40 A. 35 30 y = -0.35x + 34.533 Temperature 25 °C 20 y = -0.04x + 23.41 15 10 2 4 6. 8 Time (mins) Red=Temp as 5o mL of warm water slowly cooled over tim Green = Temp ar a mixture of both wam ahd cool water slouly cooled over me Rlue =Temp as 5o mL of cool water stayed relatively Ohstant over time
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 103AE: The bomb calorimeter in Exercise 102 is filled with 987 g water. The initial temperature of the...
Related questions
Question
Calculate the amount of energy (in J) gained by the cool water when it was mixed with the warm water at 4 minutes.
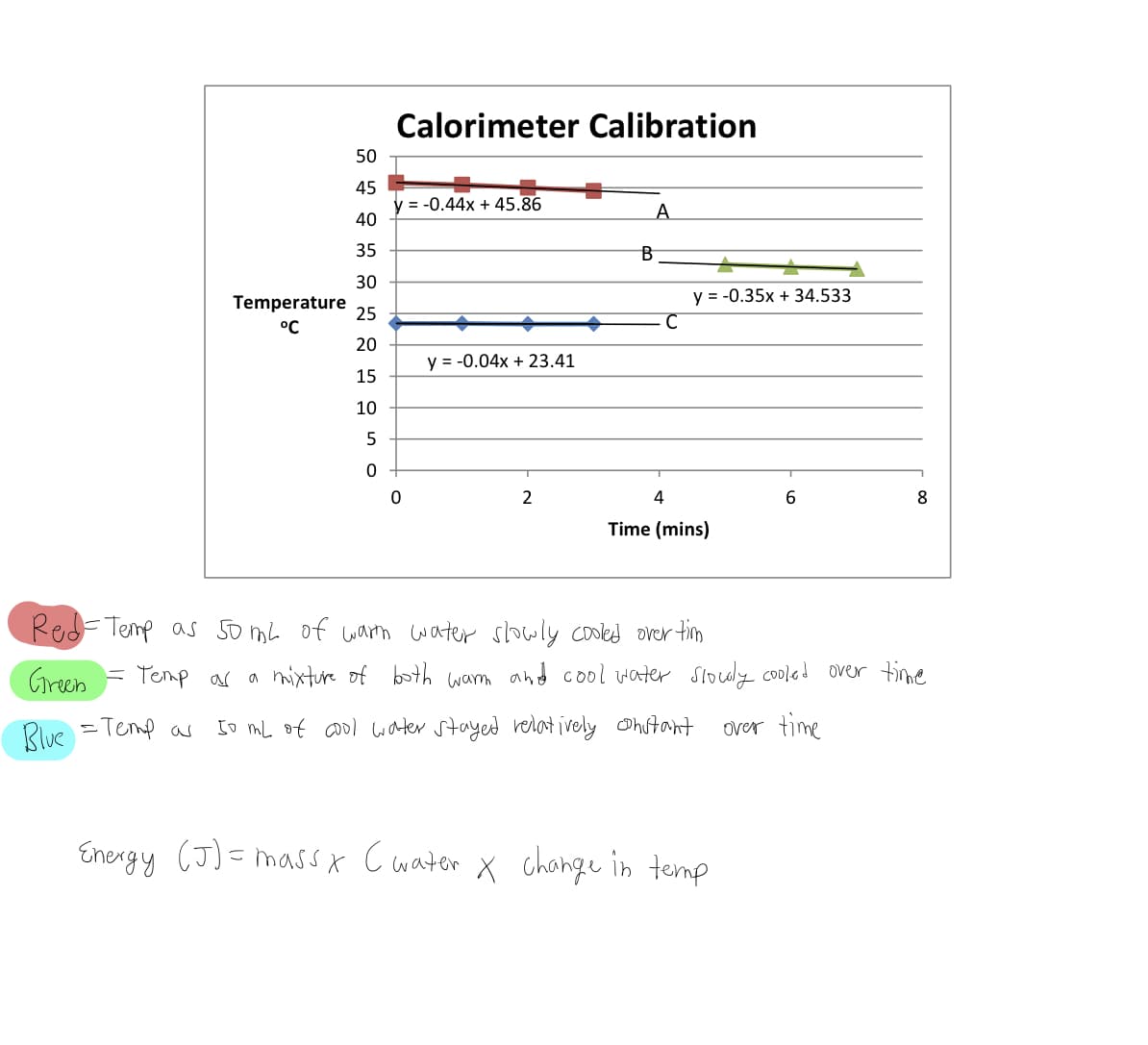
Transcribed Image Text:Calorimeter Calibration
50
45
y = -0.44x + 45.86
40
A.
35
B
30
y = -0.35x + 34.533
Temperature
25
°C
20
y = -0.04x + 23.41
15
10
5
2
4
6
8
Time (mins)
Red=Temp as 5o mL of warn water slowly cooled over tim
Green = Temp as a mixture of both wam and cool water Slouly cooled over tme
Rlue =Temp as
[o mL of co0l water stayed relat ively Ohutant
over time
Energy (J)= massx C water X chonge in temp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning