A 5.00 g piece of fäed chicken is placed into the ignition container of a bomb calorimeter then charged with excess oxygen. The bomb is placed into 2.000 L of water which is equilibrated to 24.300 °C. Previously, the calorimeter constant for this bomb calorimeter was determined to be 3.125 kJ/°C. After the fried chicken sample was ignited, the water temperature rose to 31.009° C. Determine the number of Calories in a 100.0 g sample of fried chicken. Given1 Cal is 4184 J and the specific heat of water is 4.184 J/g °C. A Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 10 Type your numeric answer and submit
A 5.00 g piece of fäed chicken is placed into the ignition container of a bomb calorimeter then charged with excess oxygen. The bomb is placed into 2.000 L of water which is equilibrated to 24.300 °C. Previously, the calorimeter constant for this bomb calorimeter was determined to be 3.125 kJ/°C. After the fried chicken sample was ignited, the water temperature rose to 31.009° C. Determine the number of Calories in a 100.0 g sample of fried chicken. Given1 Cal is 4184 J and the specific heat of water is 4.184 J/g °C. A Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 10 Type your numeric answer and submit
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 88QAP: A sample of sucrose, C12H22O11, is contaminated by sodium chloride. When the contaminated sample is...
Related questions
Question
Question attached
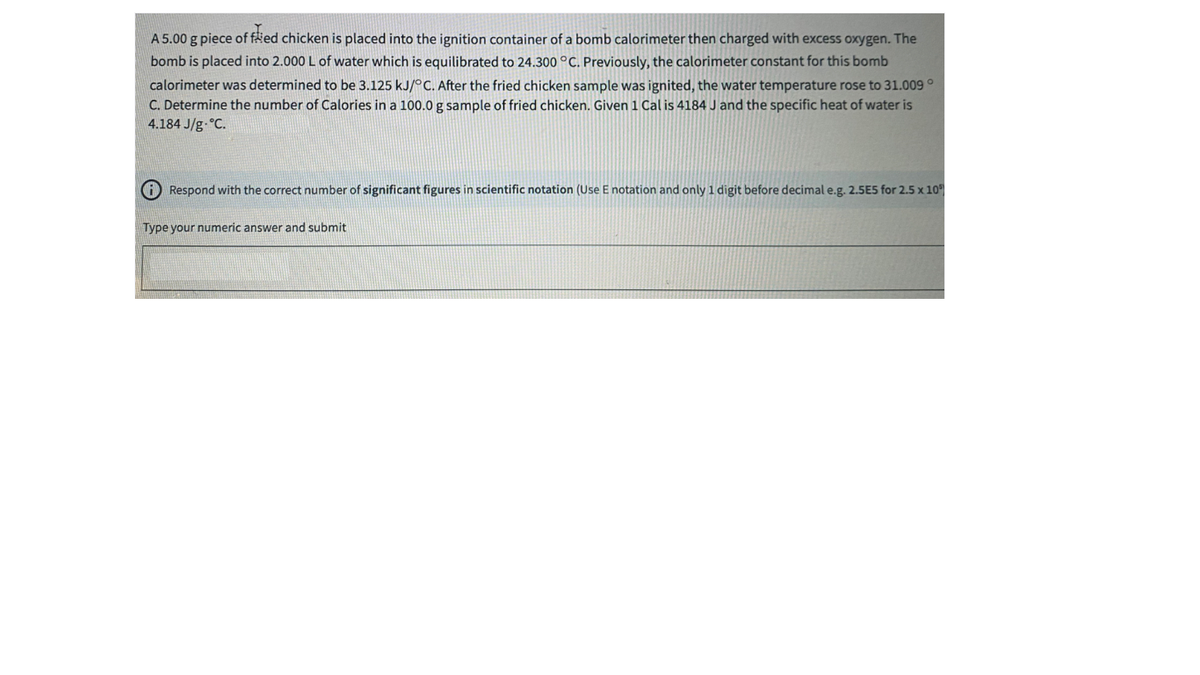
Transcribed Image Text:A 5.00 g piece of fäed chicken is placed into the ignition container of a bomb calorimeter then charged with excess oxygen. The
bomb is placed into 2.000 L of water which is equilibrated to 24.300 °C. Previously, the calorimeter constant for this bomb
calorimeter was determined to be 3.125 kJ/°C. After the fried chicken sample was ignited, the water temperature rose to 31.009°
C. Determine the number of Calories in a 100.0 g sample of fried chicken. Given1 Cal is 4184 J and the specific heat of water is
4.184 J/g °C.
A Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 10
Type your numeric answer and submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning