Can someone Outline a chemical reaction for the formation of the compound and explain it and detail I really need to understand what is going on in the mechanism
Can someone Outline a chemical reaction for the formation of the compound and explain it and detail I really need to understand what is going on in the mechanism
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
Can someone Outline a
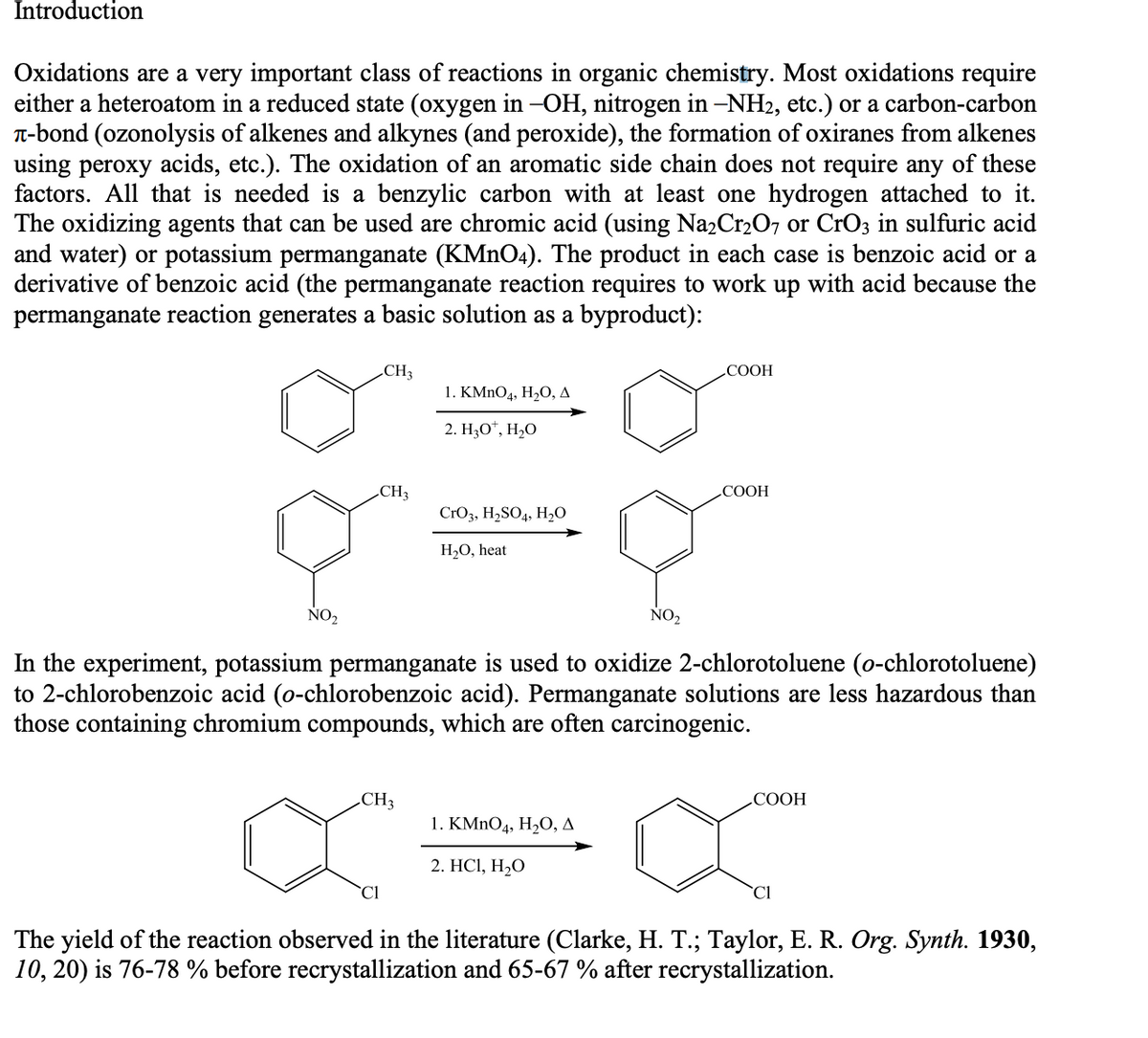
Transcribed Image Text:Introduction
Oxidations are a very important class of reactions in organic chemistry. Most oxidations require
either a heteroatom in a reduced state (oxygen in -OH, nitrogen in –NH2, etc.) or a carbon-carbon
T-bond (ozonolysis of alkenes and alkynes (and peroxide), the formation of oxiranes from alkenes
using peroxy acids, etc.). The oxidation of an aromatic side chain does not require any of these
factors. All that is needed is a benzylic carbon with at least one hydrogen attached to it.
The oxidizing agents that can be used are chromic acid (using Na2Cr2O7 or CrO3 in sulfuric acid
and water) or potassium permanganate (KMNO4). The product in each case is benzoic acid or a
derivative of benzoic acid (the permanganate reaction requires to work up with acid because the
permanganate reaction generates a basic solution as a byproduct):
CH3
СООН
1. КMnOд, Н,О, д
2. Н,О", Н,О
CH3
COOH
CrO3, H,SO4, H,O
H2O, heat
NO2
NO2
In the experiment, potassium permanganate is used to oxidize 2-chlorotoluene (o-chlorotoluene)
to 2-chlorobenzoic acid (o-chlorobenzoic acid). Permanganate solutions are less hazardous than
those containing chromium compounds, which are often carcinogenic.
CH3
СООН
1. KMnOд, H,О, д
2. HСІ, Н-О
`Cl
`Cl
The yield of the reaction observed in the literature (Clarke, H. T.; Taylor, E. R. Org. Synth. 1930,
10, 20) is 76-78 % before recrystallization and 65-67 % after recrystallization.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning