Can you explain the concept behind the problem? Can you explain where the numbers came from? And if it's possible can you provide another example. Can you show the calculations in great detail please
Can you explain the concept behind the problem? Can you explain where the numbers came from? And if it's possible can you provide another example. Can you show the calculations in great detail please
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 7P
Related questions
Question
Can you explain the concept behind the problem?
Can you explain where the numbers came from? And if it's possible can you provide another example.
Can you show the calculations in great detail please
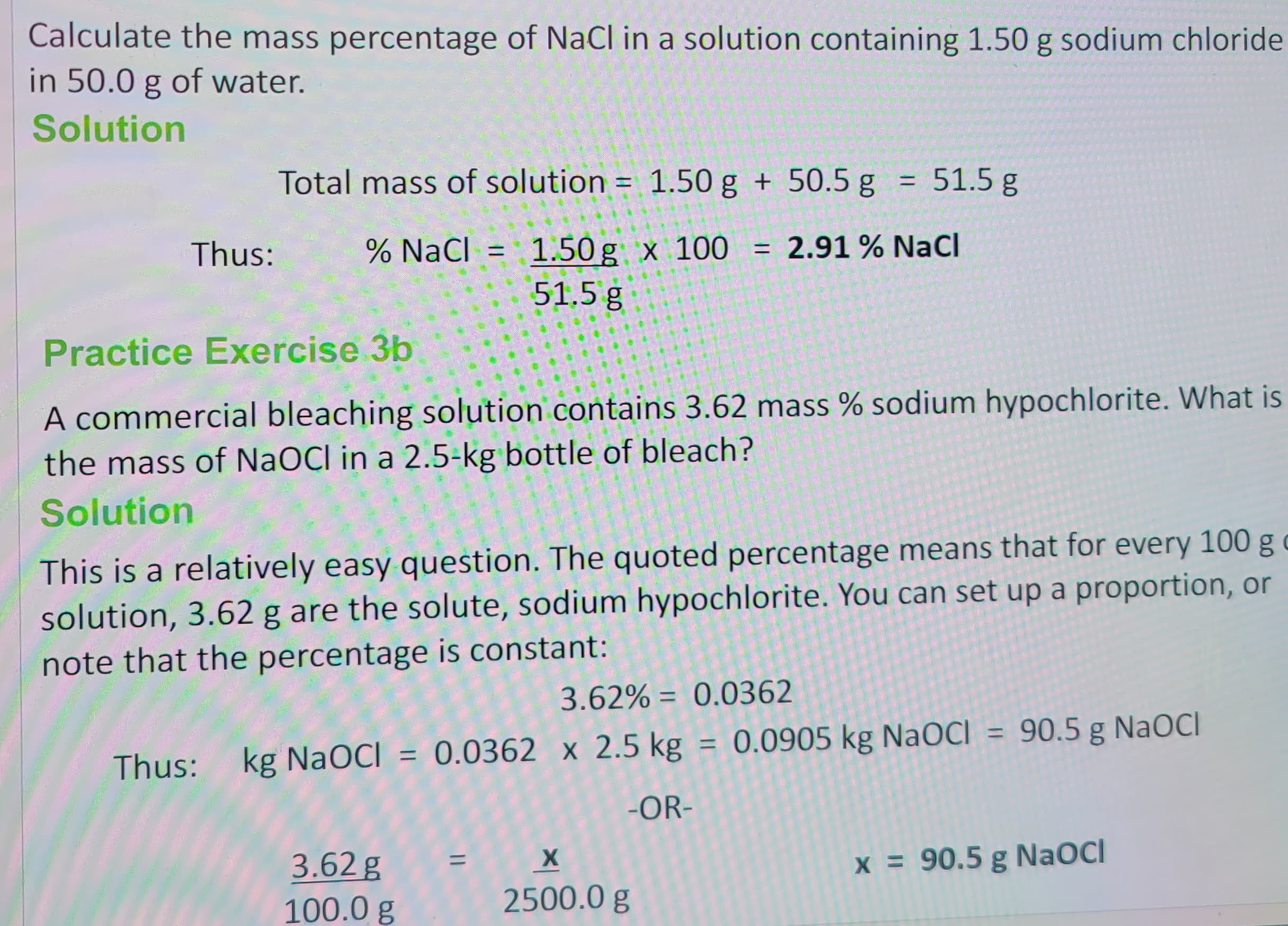
Transcribed Image Text:Calculate the mass percentage of NaCl in a solution containing 1.50 g sodium chloride
in 50.0 g of water.
Solution
Thus:
Total mass of solution = 1.50 g + 50.5 g = 51.5 g
% NaCl = 1.50g x 100 = 2.91 % NaCl
51.5 g
Practice Exercise 3b
A commercial bleaching solution contains 3.62 mass % sodium hypochlorite. What is
the mass of NaOCI in a 2.5-kg bottle of bleach?
Solution
This is a relatively easy question. The quoted percentage means that for every 100 g c
solution, 3.62 g are the solute, sodium hypochlorite. You can set up a proportion, or
note that the percentage is constant:
3.62% = 0.0362
Thus: kg NaOCI = 0.0362 x 2.5 kg = 0.0905 kg NaOCI = 90.5 g NaOCI
-OR-
3.62 g
100.0 g
X
2500.0 g
x = 90.5 g NaOCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

