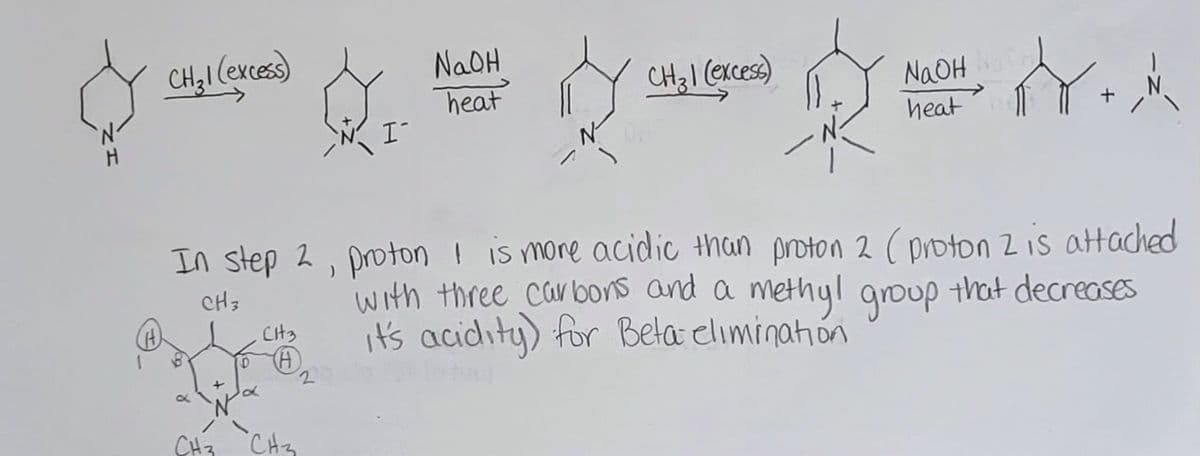
Can you please check my work on the following ochem reaction scheme and let me know if it is correct or what is wrong... the question was: Consider 3,4-dimethylpiperidine being subjected to the following: Step 1: CH3I (excess); Step 2: NaOH, heat Step 3: CH3I (excess); Step 4: NaOH, heat Provide the bond line structures for the major organic product obtained in each step and discuss the regiochemistry for Step 2.
Can you please check my work on the following ochem reaction scheme and let me know if it is correct or what is wrong... the question was: Consider 3,4-dimethylpiperidine being subjected to the following: Step 1: CH3I (excess); Step 2: NaOH, heat Step 3: CH3I (excess); Step 4: NaOH, heat Provide the bond line structures for the major organic product obtained in each step and discuss the regiochemistry for Step 2.
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 9P
Related questions
Question
Can you please check my work on the following ochem reaction scheme and let me know if it is correct or what is wrong...
the question was:
Consider 3,4-dimethylpiperidine being subjected to the following:
Step 1: CH3I (excess);
Step 2: NaOH, heat
Step 3: CH3I (excess);
Step 4: NaOH, heat
Provide the bond line structures for the major organic product obtained in each step and discuss the regiochemistry for Step 2.
Transcribed Image Text:CH₂1 (excess)
8
CH3
CH3
(A)
CH3
I-
In step 2, proton I is more acidic than proton 2 (proton 2 is attached
with three carbons and a methyl group that decreases
it's acidity) for Bela elimination
CH 3
201
2
NaOH
heat
CH₂ 1 (excess)
NaOH
heat
NY.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning