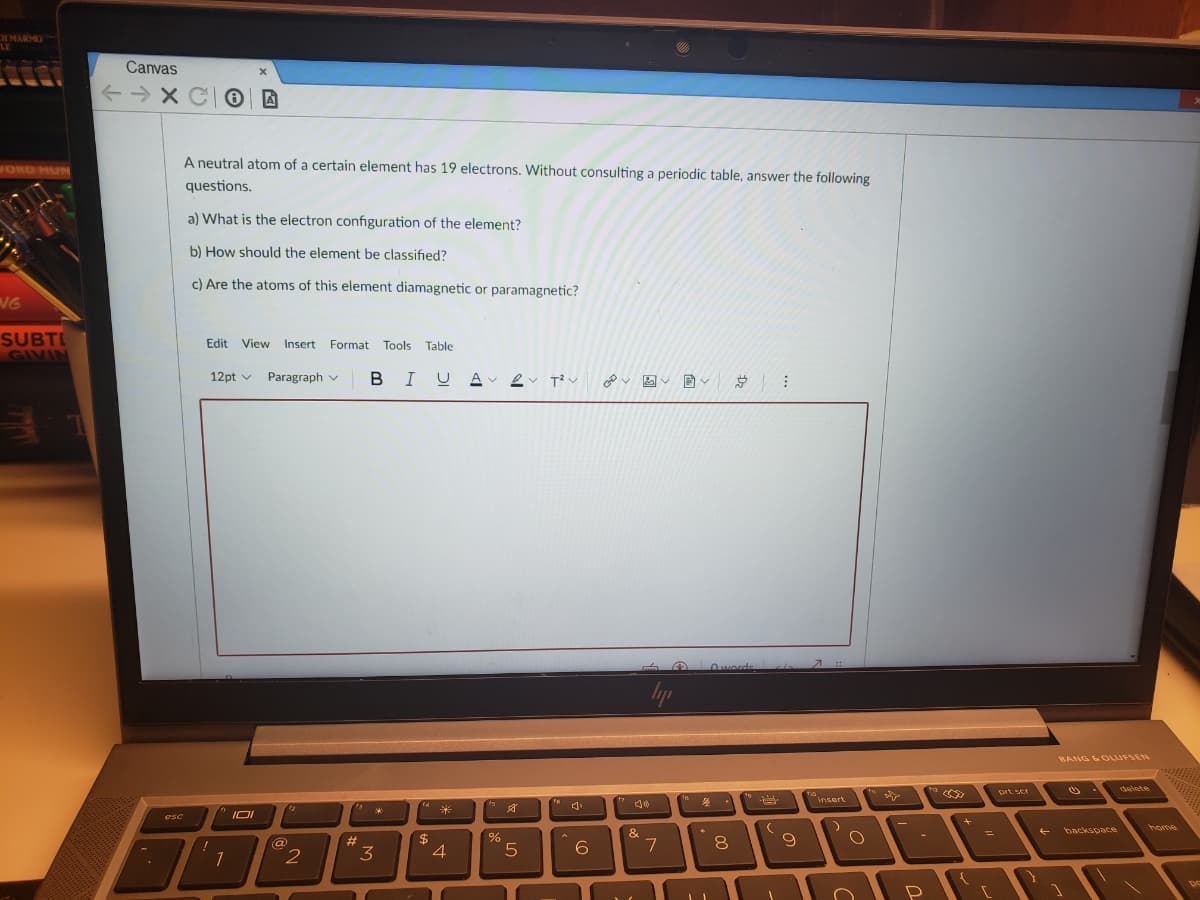
Canvas A neutral atom of a certain element has 19 electrons. Without consulting a periodic table, answer the following questions. a) What is the electron configuration of the element? b) How should the element be classified? c) Are the atoms of this element diamagnetic or paramagnetic? Edit View Insert Format Tools Table 12pt v Paragraph v BIUA 2 Owerds BANG LOLUFSEN
Canvas A neutral atom of a certain element has 19 electrons. Without consulting a periodic table, answer the following questions. a) What is the electron configuration of the element? b) How should the element be classified? c) Are the atoms of this element diamagnetic or paramagnetic? Edit View Insert Format Tools Table 12pt v Paragraph v BIUA 2 Owerds BANG LOLUFSEN
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 11ALQ: Consider the following statement "The ionization energy for the potassium atom is negative, because...
Related questions
Question
Please help
Transcribed Image Text:CH MARMO
LE
Canvas
A neutral atom of a certain element has 19 electrons. Without consulting a periodic table, answer the following
HORD HUN
questions.
a) What is the electron configuration of the element?
b) How should the element be classified?
c) Are the atoms of this element diamagnetic or paramagnetic?
NG
SUBTL
GIVIN
Edit View Insert
Format Tools Table
12pt v
Paragraph v
BIU
Owerds
lyp
BANG 6OLUFSEN
delete
prt ser
星
insert
米
esc
IO
backspace
home
%
$4
4
@
#3
3
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning